A drug eliminates aggressive cancers in a small clinical trial
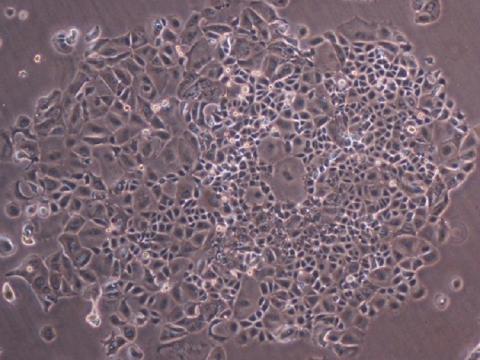
Over the past 20 years, a class of cancer drugs known as CD40 agonist antibodies has shown great potential, but also limited impact in patients and adverse reactions. In 2018, it was demonstrated that they could be improved to boost their effectiveness and limit serious side effects. A study published in Cancer Cell reports the results of using one of these drugs in a small phase 1 clinical trial: out of 12 patients, all with different types of metastatic cancer, six saw their tumors shrink, including two in whom they disappeared completely.
Luis Álvarez Vallina - cáncer CD40
Luis Álvarez-Vallina
Head of the Clinical Research Unit in Cancer Immunotherapy at CNIO-HMarBCN
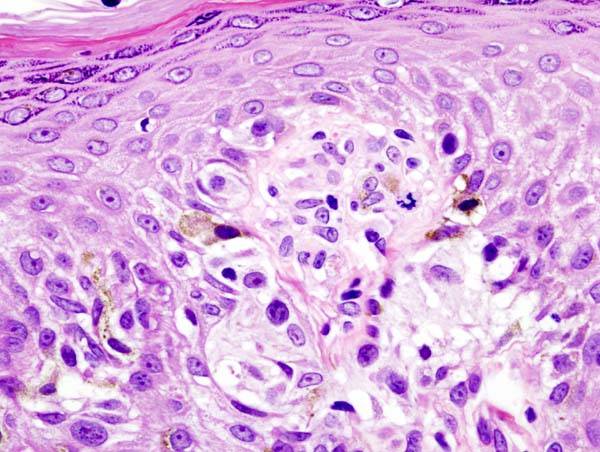
Immunostimulatory antibodies represent a promising strategy to increase response rates to immunotherapy. However, most approaches evaluated to date have been limited by severe toxicities. This study presents a novel format of immunostimulatory antibody, a CD40 agonist (named 2141-V11), designed to optimally bind to the inhibitory Fc fragment receptor of the antibody (known as FcγRIIB) and administered directly into the tumor (intratumoral). This route of administration reduces the systemic toxicity observed with previous CD40 agonist antibody formats and enhances the local activation of dendritic cells and T lymphocytes.
In a phase 1 trial involving 12 patients with advanced solid tumors, 2141-V11 showed a favorable safety profile and preliminary antitumor activity. Tumor reductions were observed in 50% of patients, with two complete responses (melanoma and breast cancer). The treatment also induced regression of non-injected lesions, associated with systemic activation of CD8+ T lymphocytes.
The study provides a strong mechanistic basis using humanized CD40/FcγRs transgenic mice.
The strategy could be applied to different tumor types, particularly those accessible to local injection (skin, lymph nodes, bladder, breast). The ability to induce tertiary lymphoid structures (TLS) and activate CD8+ T lymphocytes could synergize with, or enhance, the effect of immune checkpoint blocking antibodies, cancer vaccines, or other therapeutic strategies.
The main limitations are the small sample size and the heterogeneity of tumor types, which limit the robustness and generalizability of the efficacy signals. The maximum tolerated dose was not reached, so the optimal phase 2 dose has yet to be defined. In addition, the requirement for lesions accessible to injection restricts applicability to certain patients. Although TLS formation correlates with response, the precise determinants of their induction and their variability across tumor types remain unknown. Longer follow-up is needed to confirm the durability of responses and to define biomarkers that could better guide patient selection.
Osorio et al.
- Research article
- Peer reviewed
- Clinical trial
- People