University Clinical Hospital of Santiago
If you are the contact person for this centre and you wish to make any changes, please contact us.
Member of SEEDO and Head of Epigenomics in the Endocrinology and Nutrition Group of the Epigenomics Unit at the Santiago Health Research Institute (IDIS), Santiago University Hospital Complex (CHUS)
Head of the Paediatrics Department of the Hospital Clínico Universitario de Santiago. Coordinator of the WHO Collaborating Centre for Vaccine Safety in Santiago de Compostela and member of the WHO-Europe Vaccine Advisory Committee.
Head of the Immunology Department at the Complejo Hospitalario Universitario de Santiago de Compostela (CHUS), Servicio Gallego de Salud (SERGAS)
The Karolinska Institute has awarded the Nobel Prize in Medicine or Physiology to Katalin Karikó and Drew Weissman for their groundbreaking discoveries, which have radically changed our understanding of how mRNA interacts with our immune system, and made it possible to develop vaccines at unprecedented speed during the covid-19 pandemic.

The European Medicines Agency (EMA) has licensed Hipra's vaccine - currently called Bimervax - against SARS-CoV-2 as a booster in people aged 16 years and older who have previously been vaccinated with mRNA vaccines. The EMA began the ongoing evaluation of the vaccine, which was expected to be approved in the middle of last year, on 29 March 2022.
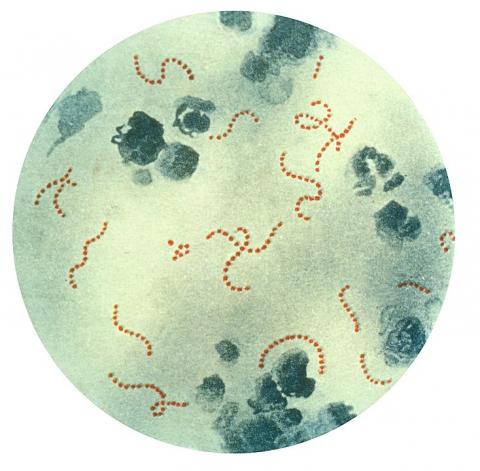
In recent weeks, the UK Health Safety Agency has detected an unusual increase in infections with Streptococcus pyogenes bacteria. At least 13 children under the age of 15 have died from this pathogen, which is responsible for mild infections but also for more serious conditions such as invasive disease. The Spanish Society of Paediatric Infectious Diseases has reported that there have been some deaths in Spain - the Community of Madrid has reported two deaths - and is analysing whether there has been an unusual increase in cases.
During Pfizer's appearance before the European Parliament this week, a company executive responded to the question of whether "Pfizer's vaccine was tested to stop transmission of the virus (SARS-CoV-2) before going to market" by saying "no".
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has approved an updated version of the covid-19 vaccine manufactured by Moderna that also targets the omicron variant. It is intended to serve as a booster dose for adults.

Según datos de la OMS y UNICEF, 25 millones de niños no han recibido sus vacunas contra la difteria, el tétanos y la tosferina (DTP) a nivel global. Se trata de la mayor caída continuada en cobertura vacunal en casi treinta años.

In a press release published this week, Moderna has shared the first data on its new bivalent adapted vaccine against Omicron.

Respiratory Syncytial Virus (RSV) was responsible for more than 100,000 deaths in children under the age of five worldwide in 2019, a study published in The Lancet estimates. The authors highlight the "urgent need" for vaccines.

The European Medicines Agency (EMA) and the European Centre for Disease Prevention and Control (ECDC) consider it too early for the general population to receive a fourth dose of covid-19 mRNA vaccines. Both agencies do agree that people over 80 years of age should receive it because of their increased risk of severe disease.

The director of the Vaccine Strategy of the European Medicines Agency (EMA), Marco Cavaleri, told a press conference on 11 January that "it is not sustainable in the long term to continue giving booster doses every three or four months [to the general population]". Spanish immunologists agree that this is not the right thing to do. In these reactions they explain why.