Reactions to clinical trial of Parkinson's drug to slow down ALS
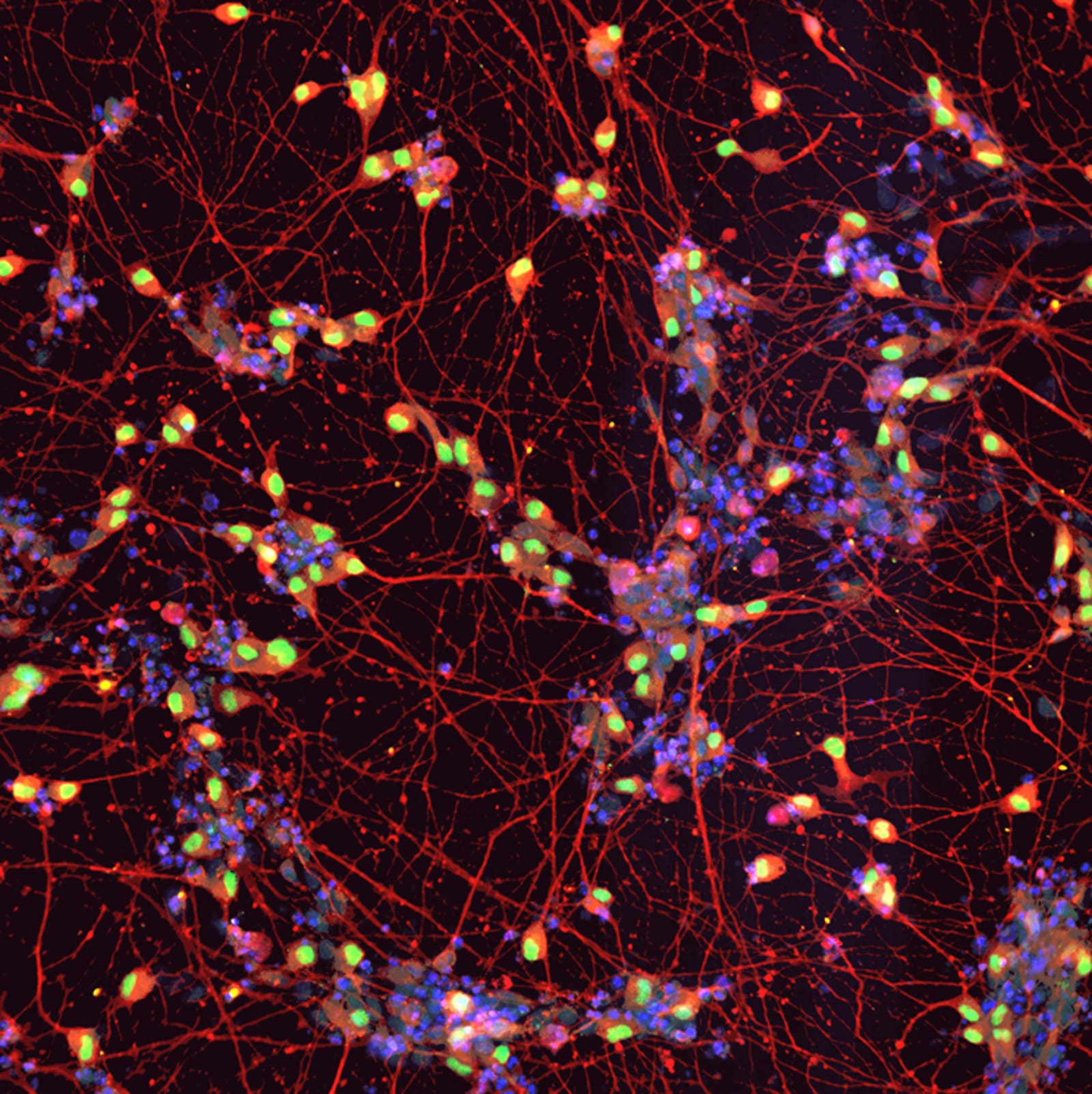
A research team in Japan has published a small clinical trial in 20 people with amyotrophic lateral sclerosis (ALS) of a drug called ropinirole, which is commonly used in patients with Parkinson's disease. The authors, whose study is published in Cell Stem Cell, say the treatment is safe and slowed the progression of ALS - an incurable neurodegenerative disease - by an average of 27.9 weeks.
Michael Swash - fármaco ELA EN
Michael Swash
Professor of Neurology, Barts and the London School of Medicine
This is an interesting report. There has long been an effort to find an “off the shelf medication" applicable to ALS management. Ropinirole may be one such drug. But there needs to be a clearer understanding of its mechanism of action in order to apply such knowledge more widely. In addition, a larger study is required to understand who might benefit and what might be the limits of practical therapy in using ropinirole in ALS. More data on possible unwanted effects are also required. There are interesting parallels in proteinaceous accumulation in the neuronal cytosol between PD and ALS that should be explored.
Brian Dickie - ELA EN
Brian Dickie
Director of Research at the MND Association
Whilst these results may be of some interest to the research community, the clinical trial is far too small and the findings too preliminary to draw any valid conclusions.
Satoru Morimoto et al.
- Research article
- Peer reviewed
- Clinical trial
- People



