Marqués de Valdecilla University Hospital (Cantabria)
If you are the contact person for this centre and you wish to make any changes, please contact us.
Head of the Neurology Department at the Marqués de Valdecilla-IDIVAL University Hospital and Associate Professor of Medicine in the Department of Medicine and Psychiatry at the University of Cantabria
Scientific Director of the Valdecilla Health Research Institute (IDIVAL), Head of the Immunology Department, and Professor of Immunology at the University of Cantabria-Marqués de Valdecilla University Hospital
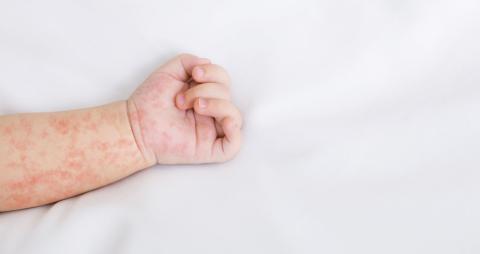
Measles cases in Europe and Central Asia fell in 2025 compared with 2024, according to preliminary data reported by 53 countries in the World Health Organization (WHO) European Region. This decline aligns with the preliminary figures published this week by the European Centre for Disease Prevention and Control (ECDC). According to the WHO, countries in Europe and Central Asia reported 33,998 measles cases in 2025, representing a decrease of nearly 75 % compared with the 127,412 cases recorded in 2024. In Spain, however, the number of cases has increased, as shown by data from the Carlos III Health Institute. A few weeks ago, the WHO announced that Spain had lost its measles-free status.

Physical inactivity is a risk factor for the onset of Alzheimer's disease. An international team has studied nearly 300 people with preclinical Alzheimer's—without symptoms but with an accumulation of tau and beta-amyloid proteins in the brain—for 14 years to find out whether physical exercise can also influence its progression. The results indicate that even very moderate activity—walking between 3,000 and 5,000 steps per day—was associated with slower deterioration, while the benefits—which appear to be related to lower tau protein deposits—were greater and tended to stabilise with activity involving between 5,000 and 7,500 steps. The results are published in the journal Nature Medicine.
A US team has analysed the presence of beta-amyloid deposits – which are linked to Alzheimer's disease – in the post-mortem retinas of four people with covid-19 and found that they were larger than in four people without covid. In complementary experiments, the SARS-CoV-2 spike protein in retinal organoids produced an increase in deposits, while the use of a drug that blocks the virus from binding to neurons reduced their accumulation. The results are published in the journal Science Advances.
An international team with Spanish participation has analysed the usefulness of a blood biomarker - the p-tau217 protein - for detecting Alzheimer's disease in 1,767 patients. According to the authors, who publish the results in the journal Nature Medicine, the test has detected the disease with high reliability in four hospital cohorts, as well as in a primary care cohort. They add that it is an assay that can be easily implemented in clinical laboratories and is already routinely used in some centres in Spain.
The DIAN-TU platform is an initiative to test Alzheimer's disease treatments early, by recruiting people with a mutation that leads to developing the disease in the future. One of the trials with the anti-amyloid drug gantenerumab ended without reaching the targets. However, a continuation of the study in 73 patients suggests - for the first time, according to the authors - that long-term, high-dose treatment given some time before symptoms develop could delay the onset of the disease. The results are published in the journal The Lancet Neurology.
A review of 14 studies and data from more than 130 million patients has found an association between the use of drugs such as anti-inflammatory drugs, antibiotics and vaccines and a reduced risk of dementia. The authors recall that “the fact that a particular drug is associated with an altered risk of dementia does not necessarily mean that it causes or helps against it.” However, “pooling these huge health data sets provides a source of evidence that can help us decide which drugs to try first.” The research is published in the journal Alzheimer's and Dementia: Translational Research & Clinical Interventions.

A study by Italian researchers has tested a treatment administered as a nasal spray to slow down early-stage Alzheimer's disease. Administered in mice, the treatment inhibits an enzyme linked to the disease and to insulin resistance. According to the researchers, who publish the work in the journal PNAS, application of the spray to the animals “can counteract the accumulation of harmful proteins in neurons and delay the onset and progression of cognitive decline”.

The European Medicines Agency (EMA) has recommended not granting marketing authorization for Leqembi™ (lecanemab) for the treatment of Alzheimer's disease. The EMA's Committee for Medicinal Products for Human Use (CHMP) considers that its effect in delaying cognitive decline does not outweigh the risk of serious side effects associated with the drug, in particular swelling and possible bleeding in patients' brains. Leqembi™ was approved in 2023 in the United States.

A family of more than 1,000 members with origins in Colombia has a mutation called "paisa" that leads to the development of Alzheimer's disease. In 2019, an added mutation in the apoE gene called "Christchurch" was described as conferring strong protection to an individual carrying two copies of it. Now, a study has found that 27 family members carry a single copy and that it is also associated with some degree of protection. According to the authors, who publish their findings in the journal NEJM, the discovery could be used to develop new treatments for the disease.
Genetic forms of Alzheimer's are considered to be those in which certain variants of a gene inevitably lead to the disease over time. Until now, only rare alterations in three genes were considered as such. A group of researchers led by the Hospital de Sant Pau in Barcelona has proposed a new, much more frequent form. After analysing data from more than three thousand donated brains and clinical data from more than ten thousand patients, they found that almost all people who carry two copies of the ApoE4 variant in the ApoE gene, which was previously only considered a risk factor, also end up developing the disease.They publish the results in the journal Nature Medicine.