Reaction to the detection of the CH3+ cation in the interstellar medium with the James Webb Telescope
A research team describes in Nature the presence outside the solar system of CH3+, a cation that could react with other molecules to form complex organic molecules. Its role in interstellar organic chemistry was described decades ago, but until now it had not been observed outside the solar system. The team, which includes co-authors from the CSIC's Institute of Fundamental Physics and the National Astronomical Observatory in Madrid, based their work on observations from James Webb Telescope.

Joan Enrique Romero - catión Webb EN
Joan Enrique-Romero
Postdoctoral researcher at the Leiden Institute of Chemistry, Leiden University (The Netherlands)
This work indicates that gas-phase chemistry may be important in protoplanetary discs irradiated by nearby stars, as it could contribute to the formation of complex molecules, the so-called 'interstellar complex organic molecules', which are considered to be the 'building blocks' needed to form prebiotic molecules.
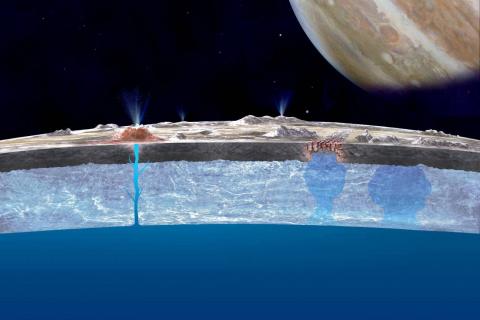
The paper presents new observations from the James Webb Space Telescope of the protoplanetary disc d203-506, in the Orion nebula cluster. The data consist of emission spectra and emission maps. The novelty lies in the possible detection of the cation CH3+ in its 'atmosphere' (the vertically outermost part), which is strongly irradiated in UV by nearby stars. Apart from CH3+ they detect other species, including CH+ and ro-vibrationally excited H2.
The importance of detecting CH3+ lies in its potential to react with other gaseous species, as its reactivity in the gas phase is well known, and the scientific consensus is that it has the ability to form more complex molecules (important for the chemical evolution of the universe), and is connected to the formation of CO through the reaction CH3+ + O, which forms HCO+ and this can form CO after reacting a free electron in the medium. CO is of special importance in astronomy, because being so abundant in cold and dense regions (in fact, it is the second most abundant molecule, after H2, in these regions), it is a perfect tracer of its physical properties.
The CH3+ assignment is based on:
- the comparison of the vibrational spectrum with a synthetic spectrum generated with computational quantum chemistry
- the spectroscopic properties expected for a molecule like CH3+.
Everything points to the assignment being correct, the methods are sufficient and the analysis timely and correct. However, like everything in 'cutting-edge science' it is not without minor drawbacks. On the emission mapping side, the authors cannot rule out contamination from the protostar jet, and high quality experimental spectra would be needed to be able to assign which lines in the vibrational spectrum belong to which emissions, and whether there are also emission lines from other chemical species mixed in with the HCO+. In any case, that CH3+ is there is the most likely scenario. In this respect, they have considered other possible species whose emission should fall roughly in the CH3+ spectral region, and there seems to be no better candidate. Finally, the detection of the rotational spectrum around 3.1-3.2 microns only makes the case that it is CH3+.
After this, the authors discuss what chemistry is expected, based on previous knowledge in the field and an astrochemical model, and compare it with what has been detected in this work, as well as previously with, for example, [the international radio telescope] ALMA. First, they discuss how CH3+ can form in that strongly irradiated environment. The film goes like this: ultraviolet photons ionise C into C+ and excite H2 rovibrationally. Thus both react to give C+ + H2* CH+. Without this excitation in H2, the reaction would be much slower, as it is endothermic. In addition, the "cold" channel of this reaction is very slow, as it involves the emission of a photon after the formation of the [CH2+]* complex, a much less efficient process. This model is supported by the H2* emission with the same JWST, and the coincidence of CH3+ and H2* emission.
Once CH+ is formed, its reaction with (not necessarily excited) hydrogen molecules is very fast, forming CH2+ and CH3+.
From astrochemical models, they obtain predictions of the abundances of various chemical species of interest as a function of 'visual extinction', i.e., as a function of how 'dark' (dense) the medium becomes, assuming different values of gas density and different sizes for dust grains, the main contributors to UV blocking. What is important about these models is that they are consistent with observations, as CH3+ follows the H2* emission, at the expected temperatures, without a strong dependence on density. However, astrochemical models in which the disc structure is also taken into account will be necessary to better understand the properties of d203-506.
Olivier Berné et al.
- Research article
- Peer reviewed