Spanish Association of Primary Care Pediatrics (AEPap)
If you are the contact person for this centre and you wish to make any changes, please contact us.
Pediatrician and collaborator of the Advisory Committee on Vaccines, the Spanish Association of Pediatrics and the Spanish Association of Primary Care Pediatrics
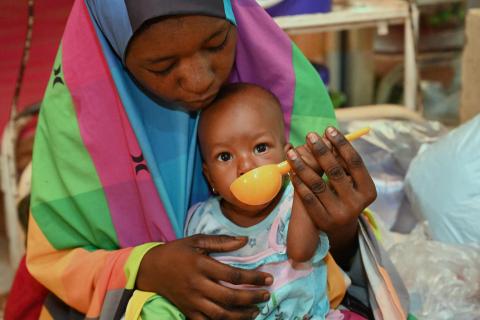
According to estimates by the United Nations Inter-Agency Group for Child Mortality Estimation (UN-IGME) published by UNICEF, in 2021 some 5 million children died before their fifth birthday, and another 2.1 million children and young people did it between the ages of 5 and 24. Additionally, 1.9 million babies were stillborn during the same period.

The European Medicines Agency (EMA) has recommended marketing authorisation in the European Union for Beyfortus (nirsevimab) to prevent respiratory syncytial virus (RSV) lower respiratory tract disease in babies and infants. The agency recommends it during its first RSV season. The European Commission now has to decide on its marketing authorisation across the EU.

Según datos de la OMS y UNICEF, 25 millones de niños no han recibido sus vacunas contra la difteria, el tétanos y la tosferina (DTP) a nivel global. Se trata de la mayor caída continuada en cobertura vacunal en casi treinta años.

Respiratory Syncytial Virus (RSV) was responsible for more than 100,000 deaths in children under the age of five worldwide in 2019, a study published in The Lancet estimates. The authors highlight the "urgent need" for vaccines.
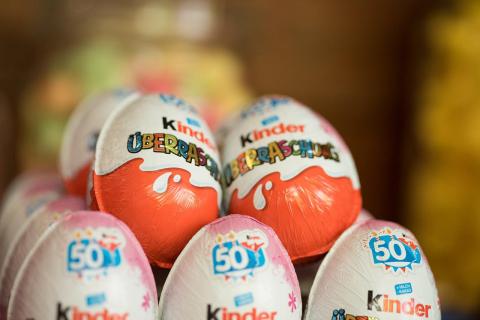
On 27 April, the WHO reported 151 cases of salmonellosis in 11 countries, caused by the consumption of 'kinder eggs' or other contaminated products of the same brand. In Spain, two cases have been confirmed.

The recommendation is to vaccinate children from 5 to 11 years old with the vaccine approved in Europe for this age group, Comirnaty (Pfizer) with one third of the dose used in children over 12 years old. But parents still have doubts. Here we answer the classic ones (safety, risk/benefit) and other more specific ones: allergies, genetic syndromes, high-risk individuals...

The United States has already approved the vaccination of children aged 5-11 years against covid-19. In Europe, the European Medicines Agency (EMA) will decide in the coming weeks on the use of the Comirnaty vaccine in this population. Here is what several Spanish paediatricians think about it.

Two studies, one published in The New England Journal of Medicine and the other, the CDC's Morbidity & Mortality Weekly Report (MMWR), find that vaccination against COVID-19 effectively protects children and adolescents aged 12-18 years from both infection and severe disease. Both papers cover periods when the more transmissible Delta variant was dominant. Paediatrician Ángel Hernández Merino assesses these results.