Nature publishes results of the Human Cell Atlas
The Human Cell Atlas, an international research consortium, publishes biological data from different cell types in the human body in a series of articles in Nature and other journals in its family. One of the articles integrates single-cell RNA sequencing datasets from the gastrointestinal tract of healthy people and others with different diseases. It describes inflammation-induced changes in stem cells that alter mucosal tissue architecture and promote increased inflammation, a concept that can be applied to other tissues and diseases.
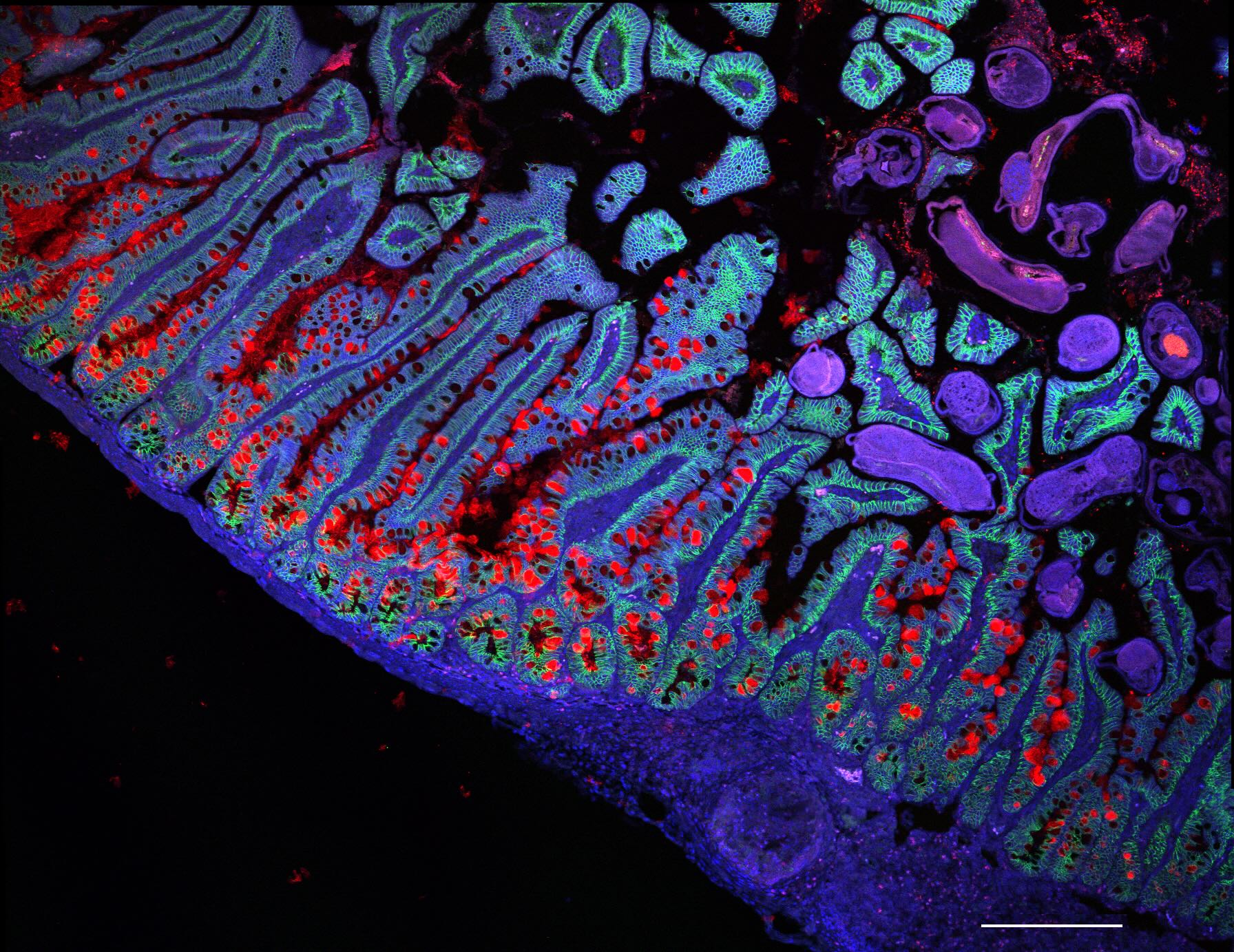
The human small intestine. Credit: Grace Burgin, Noga Rogel & Moshe Biton, Klarman Cell Observatory, Broad Institute.
241120 cell atlas iago EN
Iago Rodríguez-Lago
Gastroenterologist at the Inflammatory Bowel Disease Unit, Digestive System Department
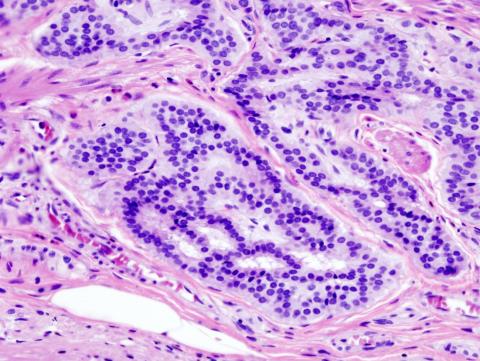
Thanks to important international collaborations, our understanding of the cellular mechanisms in healthy individuals and the pathophysiology of different diseases is being transformed. In this case, recent work brings significant advances towards the construction of the most detailed map of cell types in the human body known to date. Among the findings is the creation of an atlas of cell populations of the digestive tract, integrating data from 25 single-cell RNA-sequencing studies to map the gastrointestinal tract - an organ essential for nutrition but also notable for its role in the immune system. This atlas has combined information from healthy individuals and those with certain pathologies such as inflammatory bowel disease (ulcerative colitis and Crohn's disease) or coeliac disease, which has made it possible to identify specific cellular changes in them, highlighting the role of epithelial metaplasia associated with intestinal inflammation.
These advances are highly relevant findings for several reasons. On the one hand, because of the prevalence of these diseases, since in Spain coeliac disease affects one in 71 people in children and one in 357 in adults, while inflammatory bowel disease (IBD) is present in 0,7% of the Spanish population. In addition to this, especially for the latter, the incidence is progressively increasing and is expected to affect more than 450.000 people.
Despite their increasing frequency, the great heterogeneity of these conditions’ manifestations in each person makes them extremely complex both for their diagnosis and for the individualisation of any treatment. Therefore, the application of high-resolution techniques at the cellular level, and within international efforts such as these, are the basis for further progress in our knowledge in the coming years.
For the first time, cellular changes (metaplasia) that originate in the stem cells of the intestinal crypts and cause the cells to transform in a similar way to those found at the pyloric level or in Brunner's glands have been linked. Moreover, thanks to the possibility of analysing their function at the tissue level, their role as recruiters of T lymphocytes and neutrophils, essential for triggering the inflammatory process and causing the intestinal damage characteristic of this disease (IBD), has been further explored.
Thus, the cellular and spatial resolution of these analyses create a unique framework that allows significant progress in understanding the mechanisms behind these pathologies, as well as in the search for markers associated with the [different] manifestations and natural histories that we observe among patients, thus opening up a great opportunity to search for new treatment targets and to apply measures that are as individualised as possible to each person. These findings underline the importance of addressing inflammation at the cellular level and establish a basis for applying these insights to other inflammatory tissues and diseases, marking a milestone in the search for innovative treatments.
Beatriz Mateos - atlas células EN
Beatriz Mateos Andrés
Pre-doctoral researcher at the Integrative Genomics Lab at CIC bioGUNE (Derio, Basque Country)
The Human Cell Atlas project was born a few years ago as an ambitious international effort to deepen our knowledge of all the cells that make up the human body. After the success of the Human Genome Project at the end of the 20th century, which allowed us to solve (and still solve today) some of the great genetic unknowns about our biology and diseases, there was still a long way to go in the knowledge of how genetics intervenes in the functioning of our cells and tissues. The next logical step on this path is to investigate how these genes are activated, expressed and coordinated to give life and functionality to the cells that make up our bodies.
In this context, the aim of the human cell atlas project is to develop a detailed map of human cells. This map seeks to understand how cells change throughout life, how they alter in the context of disease, how they interact with each other, what makes them different according to organ or tissue, and even to discover hitherto unknown cell types.
This recently published study focuses specifically on the characterisation of the cells that make up our gastrointestinal system, a highly complex and extensive system with an incredible diversity of cell types. It also performs multiple essential functions for the body - from digestion and absorption of nutrients to the body's immune defence - making its characterisation a scientific challenge.
To this end, data from approximately 30 different studies have been collected, integrated and compared to provide a complete picture. This work includes data from both healthy individuals and gastrointestinal pathologies (inflammatory bowel disease, coeliac disease, etc.), which is of great value to the scientific community for several reasons.
Firstly, it highlights the importance of obtaining and analysing samples from healthy individuals. These samples are difficult to obtain, but essential since, in order to understand diseases, we must first define and characterise what health is. This project integrates multiple studies that include samples from healthy individuals, whose data will be accessible to the scientific community, and can be used for comparison with our studies. In addition, it reinforces the findings and creates a network of scientific studies that exponentially increases their power by unifying all available information on knowledge about gastrointestinal cells in a context of ‘normality’.
Moreover, accessing and integrating data from multiple diseases and different parts of the gastrointestinal system allows us to compare them simultaneously and find common patterns between them. This includes the ability to identify new, rare cell types. For example, in this paper they find a cell pattern that matches in metaplasia, inflammatory bowel disease and celiac disease, but not in healthy individuals. This information could be key in the future to identify patients at risk of progression to neoplasia.
In conclusion, thanks to single cell sequencing techniques, we are building a detailed map of gastrointestinal cells and their functions. This advance represents significant progress for the entire scientific community dedicated to the study of intestinal diseases, strengthening our knowledge and opening new possibilities to improve the diagnosis, treatment and evolution of these pathologies.
Jennifer E. Rood et al.
- Research article
- Peer reviewed
Amanda J. Oliver et al.
- Research article
- Peer reviewed
- People