Non-invasive method assesses the quality of mouse oocytes and embryos
A new technique developed at the Institut de Bioenginyeria de Catalunya (IBEC) has succeeded in assessing the health of mouse oocytes and embryos in a non-invasive way, according to a study published in PNAS. The method generates 3D images to visualise the metabolism of oocytes and embryos obtained by in vitro fertilisation and makes it possible to select those that are most likely to implant and develop, say the authors.
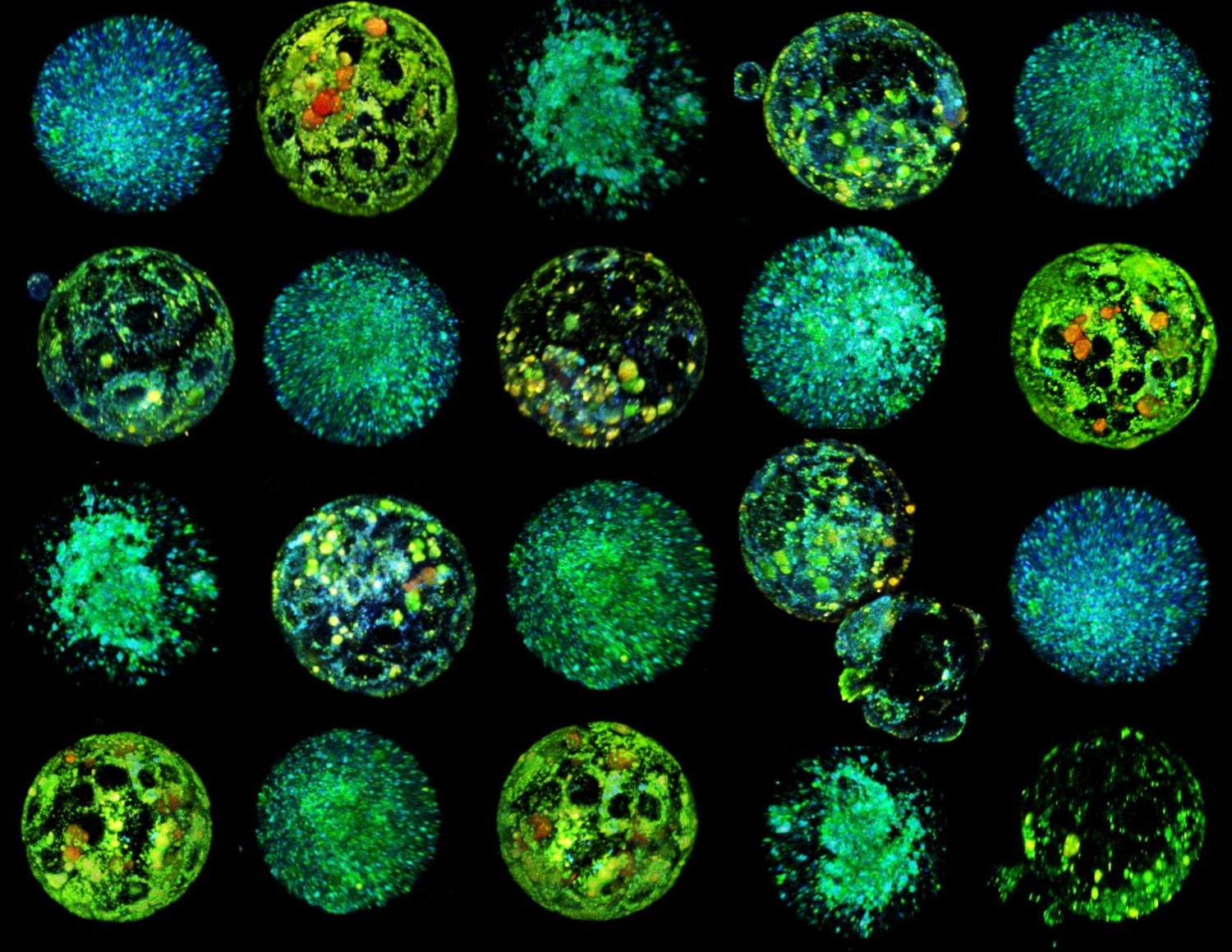
3D reconstruction of hyperspectral images of mouse oocytes and embryos. Source: IBEC.
Rocío Nuñez - técnica IBEC EN
Rocío Núñez Calonge
Scientific Director of the UR International Group and Coordinator of the Ethics Group of the Spanish Fertility Society
In this article, Albert Parra and collaborators have used, in a very well-developed study, artificial intelligence (AI) methods combined with a type of microscopy called hyperspectral microscopy (i.e. they generate images with much higher resolution than other types of microscopy), to know the quality of mouse oocytes and embryos from a metabolic point of view, without causing them harm. The model, called METAPHOR, analyses hundreds of images containing information on many metabolites in the embryos and oocytes in just a few minutes. The results have shown differences in oocyte quality as a function of age, and the authors propose that it may be of great interest in areas such as fertility preservation and personalised reproductive medicine.
However, as Ojosnegros' team [another of the authors] admits, the experiments were conducted with a limited number of samples in an animal model and more research will be needed to understand the ability to correlate METAPHOR classification with implantation ability. Researchers are currently refining the technology to screen human embryos and have created a spin-off company to bring the technology to assisted reproduction clinics in the coming years.
In assisted reproduction, there are multiple instances where AI could help improve the ability to predict live births, such as gamete and embryo selection and the development of personalised fertility drugs. While the team that developed METAPHOR demonstrates that it can be used to obtain metabolic information from live embryos and oocytes, without causing harm, there is published work that has already performed this analysis in humans. Recently, in a paper published by Sakkas et al. in which, in a rather similar way, they evaluated metabolic images of human embryos from 120 couples by fluorescence imaging microscopy, they failed to find a relationship between the pattern of metabolism of embryos that lead to pregnancy compared to those that did not.
Studies with oocytes and embryos that allow their selection and classification are promising and are progressing at an increasing rate. Parra's work may not take long to be applied in humans, but more studies are needed to know its true application in embryo selection and increased live birth rates. Overall, the widespread and rapidly growing field of AI represents a powerful and exciting opportunity for the field of reproductive biology and the IVF [in vitro fertilisation] industry.
Rita Vassena - técnica IBEC EN
Rita Vassena
Medical Director of Fertility at CooperSurgical
This study by Parra and colleagues provides a very interesting initial validation of the use of hyperspectral microscopy, a method for assessing the level of autofluorescent molecules in living cells, for the determination of viability in oocytes and embryos.
The study is carried out in mice and serves as proof of principle that this technology can indeed correctly differentiate between embryos with different metabolic profiles, a prelude to identifying those most likely to implant and lead to a viable pregnancy. In addition, the authors have convincingly demonstrated that the technology can distinguish which oocyte is likely to develop into an embryo, a feature that could be of great interest if transferred to our species.
Every year, tens of thousands of women save their oocytes for future use; being able to non-invasively know how many of them will eventually become an embryo would provide much-needed advice and peace of mind, as these women could decide, for example, to add more oocytes to their reserve or to stop at the number they already have.
This work was awaited with some expectation in the specialised scientific community; the field of assisted reproduction has been struggling for decades to find a non-invasive method to sort embryos - analysing everything from spent culture medium to time-lapse videos of developing embryos and resorting to the use of artificial intelligence in the process.
Recent attempts to use autofluorescent molecules to sort human embryos based on their ability to result in a viable pregnancy with a related technology called FLIM, while partially successful, did not reach the expected level of clinical utility.
It appears that hyperspectral microscopy, with its ability to read multiple signals simultaneously and interpolate them into a complex metabolic portrait of the embryo, may provide greater analytical granularity and the level of accuracy and sensitivity needed to demonstrate clinical utility.
Albert Parra et al.
- Research article
- Peer reviewed
- Animals