Reactions to kidney transplant from genetically modified pigs to brain-dead man
A clinical team in the US has successfully transferred kidneys from pigs to a brain-dead man. The organs came from animals that had been genetically modified to prevent rejection by the immune system of the patient, who had kidney disease. The transplanted organs were functional - they could make urine and clear creatinine - seven days after the operation, explains a research letter summarising the case in JAMA Surgery. The team says this type of xenotransplantation - from animal to human - could be a solution to the shortage of donor organs.
Toby Coates - xenotrasplante EN
Toby Coates
Professor of Medicine at the University of Adelaide and Director of Transplantation at the Royal Adelaide Hospital (Australia)
This case represents one of the first functional kidney transplants from a pig into a human, and shows proof of principle that organs from a genetically modified animal can replace human kidney function for one week without rejection and using conventional kidney transplant drug therapy.
The key advance here is the genetic removal of four pig genes that have previously proven a barrier to successful cross-species transplantation, and insertion of six human genes that prevent coagulation and 'humanise' the pig kidney to look more human-like (the 10-gene-modified pig donor).
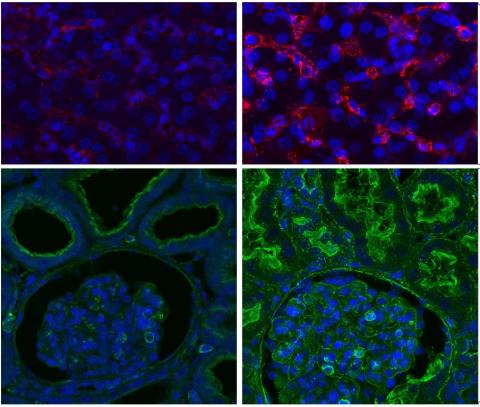
This transplant was done in a brain-dead organ donor in Maryland USA, who had their kidneys removed and the 10-gene-modified pig kidney transplanted. The transplanted kidney functioned immediately and showed no signs of rejection over a seven-day period, during which biopsies and blood tests confirmed normal kidney function.
This study confirms that genetically modified, specially housed pig kidneys can correct kidney failure and function using standard kidney transplant drugs. Although in the early phase, this pilot study provides hope for the over 15,000 Australians on dialysis who could benefit from a kidney transplant, and potentially helps overcome the shortage in human donor kidneys.
Roger Lord - xenotrasplante EN
Roger Lord
Senior Lecturer of Medical Sciences at the Australian Catholic University
Xenotransplantation is the process of transplanting donor tissue of animal origin into a human recipient.
The process has been explored for many years as a means of addressing the critical shortage of human organs available for transplantation.
Xenotransplantation has, historically, largely failed due to hyperacute rejection following surgery, even with the use of immunosuppressive drugs used to control this process.
To address this failure for kidney xenografting, investigators have removed four genes of porcine (pig) origin and added six transgene insertions to regulate the rejection process and maintain normal kidney function.
The current study involved only one brain-dead patient with chronic kidney failure as a first step to demonstrate that the 10-gene-edited pig kidneys can function normally when transplanted to remove creatinine over a 7-day period.
Creatinine is a breakdown product of creatine from muscle and protein metabolism.
Measurement of creatinine is an indication of kidney function and ability to filter waste from blood.
The investigators were also able to demonstrate that there was no evidence of microscopic blood clot formation in the xenografted kidneys which is another indicator of normal kidney function.
This case study provides important preliminary evidence that these genetically modified kidneys can function normally following xenotransplantation and offers hope to those on waiting lists for kidney transplantation.
Jayme Locke et al.
- Letter
- Case study
- People
- Animals