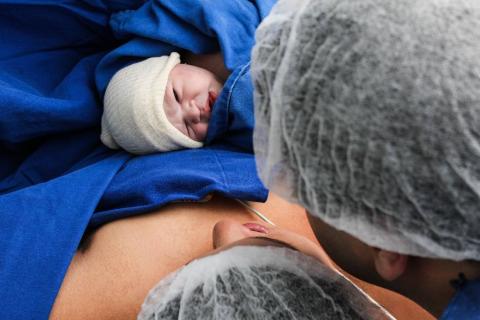
Reactions: study explores how reduced microbiota transfer between mothers and babies born by c-section is compensated
About 58.5% of a baby's microbiota come from various parts of its mother's body, according to a study of the transmission of microbes between mother and child in the first month of life. The research, published in Cell Host & Microbe, is based on samples collected from 120 mother-baby pairs, with material from their nose/throat, saliva, skin, milk, vagina and faeces. It compares babies born by caesarean section and vaginally, and confirms that the reduced transfer of faecal microbes in caesarean births can be partially compensated by other transmission routes, such as breastfeeding.
Mireia Valles - microbioma madre bebe EN
Mireia Valles-Colomer
Head of the Microbiome Research Group, Department of Medicine and Life Sciences, Pompeu Fabra University
This study complements previous ones on the transmission of the microbiome from mother to baby. We know that, during childbirth, we acquire the first bacteria that will form our microbiome, which plays a very important role in our health. As a result, babies born by caesarean receive fewer bacteria from their mothers’ gut microbiome than those born vaginally.
In this study, the authors analyse various types of samples from 120 mothers in the Netherlands (faeces, milk, skin, vaginal, saliva...) to study the contribution of these microbiomes in shaping the baby’s microbiome. In terms of the number of samples, the study extends the one published by Ferretti et al. in the same journal in 2018, in which only babies born vaginally were included. The main conclusion of the study is that the lower transmission of the mother’s gut microbiome in babies born by caesarean section may be compensated for by the mother’s other microbiomes. But in terms of methods, the technique used is problematic: the authors only sequenced part of the 16S gene of the bacteria, instead of sequencing whole genomes, as other transmission studies have done to date. Thus, the resolution obtained is limited, and it is not possible to speak unequivocally of transmission.
Regarding the press release, the title Whether born naturally or via caesarean section, babies receive essential microbes from their mothers is something we already knew: babies born via caesarean section receive fewer intestinal microbes from their mothers, but they do receive some.
Carmen Muñoz - microbioma madre bebe EN
Carmen Muñoz Almagro
Head of the Infectious Diseases and Microbiome research group and director of the Microbiology Laboratory at the Hospital Sant Joan de Déu in Barcelona.
This is a well-designed study, conducted by a research group with extensive experience in the study of the development of the human microbiome in the first months of life. The researchers demonstrate, using an appropriate methodology, how microbial transmission from mothers to their newborns is a multifactorial phenomenon, and they confirm the relevance of breastfeeding. They report that the differences in the microbiota of babies born by caesarean section and vaginal birth are not significant, since the lower microbial transfer by caesarean section is compensated by a higher transfer through breastfeeding.
Christoph Härtel - microbioma madre bebe
This is a sound and very comprehensive study in which the researchers investigated the pathways of microbial transmission from mother to child after birth. The authors of the study very carefully examined various skin and mucosal niches in 120 mother-child pairs over the first weeks of life. In doing so, they consistently checked at which niches of the child the mother’s microbes accumulate and from which resource niche they originate.
We know that C-section babies have certain risks for asthma and obesity. One reason for this could be a different microbiome than in children born vaginally. A second possible reason may be antibiotics given to the mother during the caesarean section. It was under this assumption that all the ‘vaginal seeding’ ideas came about. C-section babies lack the microbiome from the birth canal. We know that in the last four weeks before birth, the mother’s microbiome changes in the birth canal to give the baby a boost of ‘good’ bacteria at birth. If that’s missing, then the child receives the first bacteria in other way, probably mainly through the skin. Vaginal seeding, the inoculation with vaginal bacteria after birth by swabs, is a potential means to compensate for that.
Mothers of C-section babies – and their parents in general – often wonder if there is anything they can do for the child to help with its microbiome. The study provides a first positive message, which we actually always give to the women after birth: lots of cuddling, lots of breastfeeding. This compensates for the lack of exposure with vaginal bacteria. And that’s what this study now shows as well.”
Does the study allow us to say that ‘vaginal seeding’ is redundant and can be replaced by breastfeeding?
“It has not yet been proven in studies that ‘vaginal seeding’ has a long-term positive effect on, for example, the risk of asthma or obesity. Seeding has shown positive effects in the context of studies, but it also carries possible risks, such as the transmission of viruses, which is why this method has not yet been recommended by professional societies. Now we have the first proof that you don’t necessarily have to do it – that’s a relief.
The data show that caesarean babies benefit very much from breastfeeding, and much more quickly than babies delivered vaginally. And the amount of cuddling – as in contact with the mother’s skin so that the microbiome is transferred to the child – provides a higher diversity of microbes, which again is protective. It would be interesting to study whether the children who received a lot of cuddling and breastfeeding then go on to develop less asthma, for example.”
Can breastfeeding compensate quantitatively (microbiota quantity) or qualitatively (microbiota composition) for lower microbiota transmission in babies born by Caesarean?
“We see differences in the pioneer bacteria, that is, the first species of bacteria that colonise the child, because the first transmission is different. However, one cannot look at the microbiome in isolation, but must always think of it as part of a complex system, because it also interacts with the immune and metabolic system, among other things. The microbes produce metabolic products, which in turn contribute to the maturation of organs. Microbes colonise our whole body and contribute to our health. There is a specific microbiome in the lungs and in the gut: how do the bacteria communicate with each other, how do the microbes in the gut communicate with the brain? The bottom line is that you can’t say that you can probably solve all problems just by breastfeeding. Breastfeeding can have an almost one-to-one beneficial effect on gut colonisation, and it lowers the risk of asthma, but we have not yet proven that this is due to the pioneer bacteria being different in asthma patients compared to healthy individuals. This requires long-term, complex studies that also include effects on other components, such as the immune system or metabolism. But the authors also commented on these limitations.”
The authors of the study say it makes evolutionary sense for transmission pathways to be redundant. How plausible is this statement?
“Nature has designed the maturation and development of children to equip the child in a healthy way. Nature is able to adapt when a pathway cannot be taken. Another example: some newborns suffer a stroke, but nature’s plasticity can manage at this early stage of development to sprout alternative auxiliary pathways that help to take over the function of the stroke area by other brain regions. This does not exist in adults. So, it seems logical that there are different pathways and adaptations for the child to get to the microbiome it needs, even if there are risk factors, such as the mother not being able to breastfeed or being given antibiotics.
Declares that he receives funding from the German Federal Ministry of Education and Research.
Debby Bogaert et al.
- Research article
- Peer reviewed
- Observational study
- People