What are biological medicines?
A biological medicine is one that comes from living organisms or cells. They began to be developed in the 1980s and are used to treat a wide range of diseases. They include insulin, growth hormone, vaccines, and a variety of monoclonal antibodies used to treat autoimmune diseases or many types of cancer.
They are very useful drugs, but complex and expensive. Of the 20 active ingredients with the highest hospital consumption in Spain, 14 are biologics. Specifically, adalimumab, which is used in the treatment of rheumatoid arthritis, is the one that accounted for the highest expenditure in 2019, just over 372 million euros, according to data from the Ministry of Health. The antibody eculizumab - used in a rare disease - was the most expensive per patient that year, with a price tag of around 300,000 euros per year.
What are biosimilars?
A biosimilar is a medicine developed to be precisely similar to an already authorised biologic, which is called a reference medicine. Both have to be shown to be equivalent in quality, efficacy and safety. A biosimilar can only be marketed after the patent of the reference medicinal product has expired and its exclusivity period has elapsed.
What are the advantages of biosimilars and why are they being developed?
Biological medicines are complex, the process to approval is long and they are often expensive. This is a major cost to healthcare systems, which can limit their reach to all patients who need them. The entry of biosimilar medicines into the market means that they compete with the reference medicine and the price of treatment is lower.
Are they the same as generics?
No, although the concept is similar. Generics are chemically synthesised medicines whose structure is usually simple, so they can be guaranteed to be the same as the original. Biologics are much more complex molecules obtained from living organisms, so there is no guarantee that a biosimilar is exactly the same as its reference medicine. However, for the same reason, this also happens between different batches of the same original biological medicine.
How many biosimilars are approved and how much are the savings?
In Spain, 67 biosimilar medicines are currently approved, corresponding to 20 active ingredients. In Europe, 78 drugs are approved, encompassing a total of 21 active ingredients. According to a study commissioned by the Spanish Association of Biosimilar Medicines (Biosim), the use of these medicines will save around 1,048 million euros by 2022 in Spain. Between 2009 and 2022, the estimated saving was just over 5 billion euros. Biosim comprises 14 companies that develop or market biosimilars, although some of them also do so with reference medicines. According to its general manager, Encarnación Cruz, these figures "are conservative". Moreover, taking into account the number of reference drugs whose patent period is about to expire, "the susceptible market will triple by 2030," she says.
How much are they used in relation to the reference drug?
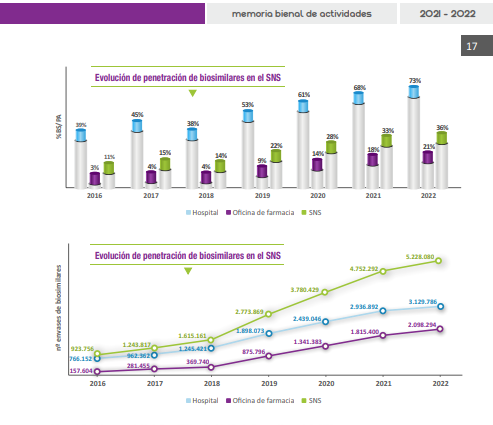
According to Biosim data, the overall penetration rate of biosimilar medicines (the proportion of total sales of active substances) is 36 %. Specifically, the percentage is 73 % for hospital use and 21 % in pharmacies. This disparity may be due to "the fact that there is a greater number of biosimilar medicines in the hospital setting and that they arrived earlier than in pharmacies, having generated greater confidence in their handling, but it is possible that in the future there will be a rapprochement between the two percentages," explains Vicente Merino, Specialist Pharmacist at the Virgen Macarena Hospital in Seville and representative of the Spanish Society of Hospital Pharmacy (SEFH).
Another aspect, Cruz points out, is that of pricing: "In hospitals they are usually purchased through public tenders and biosimilars are the ones that tend to be presented at the lowest prices. In pharmacies, the price of the original and the biosimilar is the same by law, about 30% lower than before the biosimilar was introduced". Although the cost for everyone is lower, "this measure discourages people from switching to biosimilars and creates a captive market", says Cruz, who believes this could discourage "companies' interest in developing new biosimilars".
What is the process for approval and what steps are required?
In Europe, biosimilar medicines are assessed and approved by the European Medicines Agency (EMA). Its opinion guarantees interchangeability with the reference drug [the possibility of using it instead], but actual substitution will depend on the decisions taken by each member country and the availability of the product in each region.
The assessment is somewhat different from that for originator biologics. As these are already proven safe and effective, the testing of biosimilars is more oriented towards ensuring that they are comparable and similar to the reference product. This does not mean that the process is more lax. As explained in the European Commission's guidelines, the authorisation of biosimilars requires a different set of data than for reference biological medicines, but the same high standards of quality, safety and efficacy apply.
"The preclinical studies phase (before testing in humans) is very exhaustive," explains Merino, who assures that "a very extensive battery of different analytical tests is used to ensure that they are as similar as possible and that there are no differences in efficacy, safety and immunogenicity [a response by the immune system]". As for the clinical phase, phase 1 and 3 trials are conducted and the intermediate stage can be omitted, but when they have passed the pre-clinical phase "they have proven to be very safe and have never given problems, so there is even talk of dispensing with phase 1". As for phase 3, "even more patients are usually included than in trials with the reference drug," Cruz adds.
Is it possible for them to be approved for indications for which no specific clinical trials have been done?
Yes, this concept is called extrapolation. If the reference medicinal product has proven to be useful for several indications, the biosimilar does not have to prove its efficacy for all of them in clinical trials, as safety and efficacy data can be extended. However, it is the EMA that decides on a case-by-case basis whether new studies are needed for each indication.
There have been controversies about the safety and efficacy of biosimilars: why, and are they justified?
"With biosimilars, something similar has happened to what happened with generics, which took many years to trust that they were the same as the originals," says Encarnación Cruz. From the first approvals, there was controversy about whether they were really comparable in terms of safety and efficacy, with the addition that there was a certain variability inherent to biological medicines. According to Merino, doubts also sometimes arise "from extrapolation to other indications, as sometimes they have not been specifically tested for a particular indication". And this atmosphere "may sometimes have been contributed to by certain messages from the reference drug industry itself", he adds.
However, since the first biosimilar was approved in Europe in 2006, "none has given problems in terms of efficacy and safety beyond those that the reference biologic can give, and if there has been any drawback, it has been attributed to the nocebo effect [an undesirable clinical outcome due to negative expectations generated by the patient about the biosimilar]," explains Merino. "There are many articles published on the lack of problems with biosimilars, due to the stringent approval processes," Cruz also says.
Regarding variability, a 2010 article by the Clinical Management Unit of Hospital Pharmacy at the Valme Hospital in Seville asked why the controversy arose with the appearance of biosimilars, when biological drugs were sometimes exchanged with each other before a new one came onto the market. "You can't be sure that these drugs will never have a problem. The possibility exists, but logically with a probability of occurrence equal to that of any new biotech drug that has been on the market for several years and changes its production process," they added.
What proposals are there for increasing their use and development?
These are some of the initiatives proposed by the experts consulted:
- Establish incentive mechanisms for prescribers through profit-sharing, so that the savings generated are reinvested in the same hospital centre and service. According to Cruz, this is a way of "visualising that savings lead to improvements".
- According to Merino, the SEFH proposes that hospitals "use biosimilars at the same price" as a way of encouraging their use and development. In pharmacies, Biosim proposes that the original drug cannot be sold at a discount during the first year after the introduction of the biosimilar, in order to avoid a captive market.
- Establish a target percentage of use at national level, as has been done in other European countries.
- Increase its prescription in earlier stages of diseases such as cancer. According to Cruz, biological drugs are often reserved for more advanced stages because of their price. As this price decreases with the appearance of biosimilars, this type of treatment can be extended and brought forward, "but this is often not being done".
- Carry out institutional information campaigns for the population.
Although their deployment can still be improved, Cruz does not deny that there has been a positive evolution in the acceptance of biosimilars, as "many uncertainties have disappeared, especially in the hospital setting, and their use has grown in recent years". Merino is clear that "biosimilars are here to stay, as they will make it possible to incorporate innovation while keeping the Public Health System sustainable".