The International Agency for Research on Cancer (IARC) is a body of the World Health Organisation (WHO). Established in 1965, it conducts and coordinates studies on the causes of cancer, its international surveillance and epidemiology.
The IARC regularly enters the media and public debate through its ‘monographs’ (assessments). In these, a group of international experts evaluates the available scientific knowledge —both in humans and animals— to make a qualitative assessment of the risk posed by a given chemical or biological agent or activity. As a result, it is classified into one of the four existing groups.
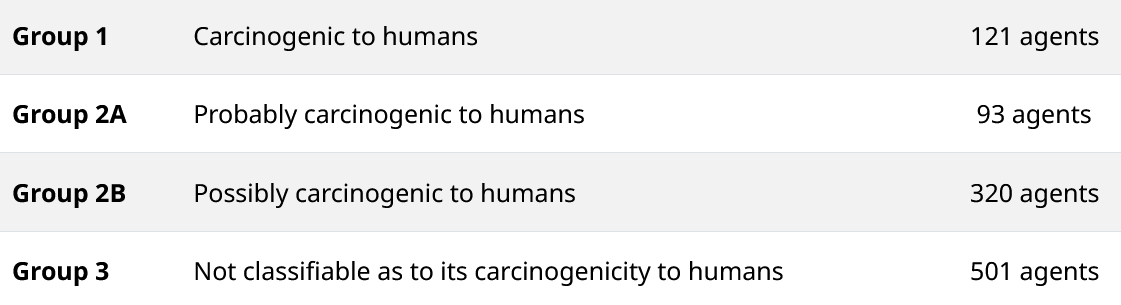
The classification only reflects the strength of the evidence
IARC monographs are qualitative, not quantitative. In other words: they reflect how certain the knowledge is that the agent is carcinogenic, not how carcinogenic it is (magnitude of risk) or how many cancers it is associated with. The task given to IARC is ‘risk identification’ –really, the identification of products or activities that constitute a hazard–, not 'risk assessment'.
Therefore, the classification does not imply that Group 1 agents are more carcinogenic than Group 2B agents. Nor does it imply that the more than 100 agents classified in group 1 pose an identical risk to each other: although processed meat and tobacco are in the same group, we know that smoking is far more dangerous than eating bacon.
Not all agents studied are the same
The agents (substances, activities) studied by IARC are very diverse. They range from single substances, such as the human papillomavirus, to more complex exposures, such as air pollution. They also include activities: for example, working in certain industries —such as the textile industry— and even the night shift.
Animal and human studies
The classification is based on epidemiological studies in humans that analyse the incidence of cancer in relation to the agent of interest, but also in animal experiments. The latter may be sufficient to move up a level in the classification on certain occasions.
Thus, an agent can be placed in group 1 even if the evidence is “less than sufficient” in humans, as long as there is “sufficient” evidence in animal models and “strong” evidence in humans.
In addition, the classification also considers the existing knowledge of one or more biological mechanisms that explain the carcinogenic effect.
The list is constantly being updated
No classification is written in stone but depends on the scientific knowledge available at any given time. Almost half of the more than 1000 agents tested to date belong to group 3. This means that the agent is “not classifiable as to its carcinogenicity to humans”. This category is used “when the evidence in humans is inadequate, the evidence in animals is limited or inadequate and the mechanistic evidence is limited or inadequate”.
It is important to note that “limited evidence of carcinogenicity in experimental animals means that the available knowledge suggests a carcinogenic effect, but that this is not conclusive”. In other words: the fact that an agent is in group 3 does not guarantee that it is not carcinogenic, but that there is insufficient knowledge and therefore that more research is needed.
The IARC is not without controversy
A wide range of researchers have explained the strengths, weaknesses and contributions of IARC assessments. IARC’s task is risk identification, not risk assessment and management, as it cannot dictate to governments what to do.
The IARC methodology does not assess the risks of exposure, the likelihood of developing cancer or the level of exposure necessary to do so.
In addition, corporations often criticise the body’s decisions to list its products in Groups 1, 2A and 2B. Thus, monographs on meat, the glyphosate pesticide and radiation from mobile phones have been controversial in the past.