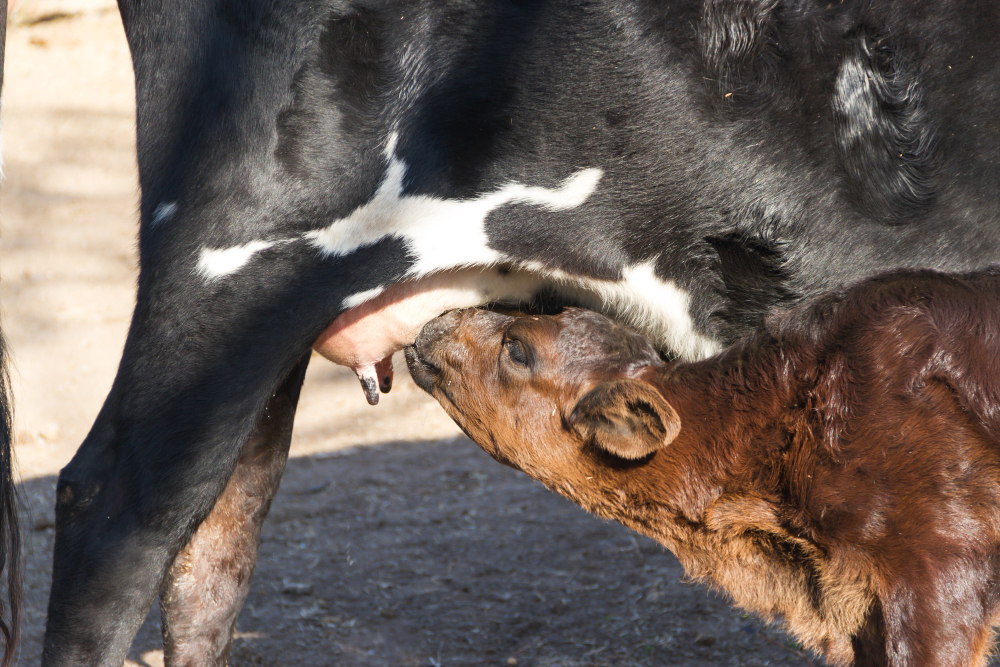
The H5N1 avian influenza virus is transmitted between mammals from contaminated cow's milk and reaches the mammary glands
The H5N1 avian influenza virus can be transmitted between mammals, according to a study published in Nature. The research team isolated the virus from the milk of an infected cow in New Mexico (USA) and found that it spreads in mice and ferrets, reaching the mammary glands of both animals. In addition, the virus was also transmitted from infected lactating mice to their offspring. The European Centre for Disease Prevention and Control (ECDC) has issued a press release on Monday recommending increased surveillance for these viruses.
Aitor Nogales - gripe leche mamíferos EN
Aitor Nogales González
Senior scientist at the CSIC at the Animal Health Research Centre (CISA), National Institute for Agricultural and Food Research and Technology (INIA)
Recently, in March 2024, an outbreak of highly pathogenic avian influenza (HPAI) subtype H5N1 was reported for the first time in dairy cows. This was the first time that these avian influenza viruses were detected in cattle. These outbreaks have spread across several US states and are in addition to those already observed across the globe in other mammals such as sea lions, cats and mink. In addition, as a consequence of the spread of the virus in livestock, infections have also been described in workers who have been in contact with infected animals, as well as other farm animals, such as cats and, of course, birds. The virus was also detected in cow's milk and it is thought that this could be a route of transmission.
In this good work by Kawaoka's prestigious group, the pathogenicity and transmission of a virus isolated from contaminated milk has been characterised. Two animal models widely used in the field, mice and ferrets, were used. Researchers have shown that animals can become infected by consuming contaminated milk and that vertical transmission occurs in suckling mice. However, the bovine H5N1 virus was transmitted inefficiently by the respiratory route in ferrets. Some of these results are similar to those obtained with other H5N1 viruses in the past. However, the researchers also point out that the virus isolated in the cattle outbreak possesses characteristics that may facilitate infection and transmission in mammals, including humans. This conclusion is mainly due to the ability of the virus to bind to cellular receptors present in the upper respiratory tract of humans.
Given the increase of cases in mammals that have been infected by avian influenza subtype H5N1 and the characteristic evolvability and adaptability of the influenza virus, the situation should continue to be actively monitored. In addition, it is important to assess the evolution of the virus and the functional significance of changes in its genome to identify possible adaptations to mammals, including humans.
Although the risk to the population, in general, is considered low, this situation could change in the future and we must remain vigilant. We must remember that recent influenza pandemics have had avian influenza as a key player.
Gustavo del Real - gripe leche mamíferos EN
Gustavo del Real
Senior scientist in the Biotechnology Department at INIA-CSIC
In March this year, the first outbreak of H5N1 highly pathogenic avian influenza in cattle was announced. It occurred in the state of New Mexico (USA) and, as of May, 69 affected herds have been reported in nine other states. In parallel, several cats on affected farms have died and there have been three cases of farmers infected with conjunctivitis and respiratory symptoms.
This is a quantum leap in the ability of these highly pathogenic H5N1 viruses to transmit to other non-poultry animal species. Until now, transmission of this type of virus to various mammalian species, especially cats, mustelids, seals, elephants and sea lions, as well as a few hundred isolated human cases, had been reported. Fortunately, subsequent sustained transmission between individuals of these species is not very efficient, with the exception of those that have occurred in recent months in marine mammals off the coasts of South America and in the Antarctic region.
The novelty of this new episode, apart from affecting the bovine species for the first time, is the presence of the virus in the milk of infected cows, which facilitates a new route of transmission of the virus both to their offspring and, potentially, to the human species - only if the milk is not properly sanitised.
According to the authors of this experimental study, conducted in mice and ferrets, the ability to transmit through milk derives from the virus's ability to proliferate in the mammary gland. This new ability of the virus may have been produced by its ability to use appropriate cellular receptors as a result of its adaptation to mammals.
This first experimental approach will open up new studies carried out in the bovine species itself to reveal the pathogenesis of the infection, as well as the possibilities of transmission to other animal species and, especially, to humans as it is a key livestock species.
Elisa Pérez - gripe leche mamíferos EN
Elisa Pérez Ramírez
Researcher in the Department of Infectious Diseases and Global Health at the Centre for Animal Health Research CISA-INIA, CSIC
This is a very solid article carried out by a group of recognised prestige in the area of research on avian influenza viruses.
The results are highly relevant for their impact on animal and public health in the context of a very relevant and highly topical health alert: H5N1 avian influenza.
Since the end of 2020, this virus has caused the death of millions of poultry worldwide and hundreds of thousands of wild birds. In fact, we are living through the largest avian influenza epidemic in history. The impact on the poultry industry and wildlife has been devastating. In addition, the virus has become progressively more capable of jumping to mammals, first in the wild (with mass mortalities of marine mammals in South America, for example), then mink in fur farms in Spain and Finland, outbreaks in domestic cats and finally, the most unexpected and probably the most worrying jump: dairy cattle in the United States. This subtype has never before been detected in ruminants, so this is a completely new scenario with many question marks. Since the end of March, when the virus was confirmed in the first herd in Texas, the virus has been spreading unstoppably across the country, affecting 141 farms in 12 states.
This is the context for this study, which attempts to answer some key questions related to the potential of the virus isolated in cows to be transmitted between mammals by different routes.
The authors confirm that the H5N1 virus in cows (belonging to a specific genotype called B3.13) can cause systemic infection in mice. Mice are easily infected both orally (by ingesting milk from infected cows) and intranasally (by inoculating the virus into the nostrils). Infection by both routes causes disease and death in a percentage of mice and the virus is distributed throughout the body, reaching the mammary glands. In fact, the authors confirm that vertical transmission can occur in the mouse model, i.e. a lactating female can transmit the virus to her offspring via milk.
In addition to mice, this study also evaluates the characteristics of infection in ferrets, which is a species commonly used in in vivo research on influenza viruses and is considered a model closer to humans. Intranasal inoculation in this species also caused disease in the animals and the virus managed to reach many organs, including the brain, eye and mammary glands. Airborne transmission between ferrets occurred, but inefficiently because only one of the "contact" animals developed antibodies, although the virus could not be detected in the nasal swabs of this individual.
Another very relevant finding of this study is that they have shown that the virus isolated in cows has a high affinity for the receptors that the virus normally uses to enter avian cells (alpha 2,3) but also for mammalian receptors (alpha 2,6). These receptors are present in the upper respiratory tract of humans, so this particular genotype of H5N1 may be more capable of infecting and transmitting between humans.
In conclusion, the results of this study demonstrate that the H5N1 subtype isolated in cows has a high tropism (affinity) for the mammary glands, where it replicates intensively, and that at least in mice, the offspring of an infected lactating female can become infected by suckling. This is very relevant in terms of food safety implications in cow's milk and as a possible route of mother-to-calf transmission in cow farms.
Furthermore, it is the first time that an avian influenza virus has the ability to bind not only to avian but also to mammalian cell receptors and, albeit inefficiently, can be transmitted by air between ferrets. All these results indicate a high potential for transmission between mammals and thus an increased pandemic potential of this particular subtype.
With this new information, the importance of keeping a close eye on the H5N1 virus is even more evident. It is never good news when a highly pathogenic virus of avian origin adapts to mammals, but in this case the situation is particularly worrying because this is a livestock species with intense interaction with humans. Faced with this risk scenario, it is imperative to support a One Health strategy in which health surveillance is intensified both in cattle and in workers who are in contact with these animals.
Ursula Höfle- gripe leche mamíferos EN
Ursula Höfle
Contracted Lecturer, member of the SaBio Group at the Institute for Game and Wildlife Research (IREC) (CSIC-UCLM-JCCM)
The H5N1 avian influenza virus currently circulating and responsible for the recent outbreaks in dairy cattle in the US has broken all the moulds of the usual avian influenza viruses, conquering new continents, affecting many new species and losing its seasonality.
The occasional jumps to mammals, including occasionally humans, have the scientific community vigilant and working on the characterisation of viruses emerging in new mammalian species to understand the risk they pose to global/human health. This work is part of these studies, which range from characterising the bovine udder epithelium and virus replication, transmission to calves or transmissibility to abundant and important mammals for the epidemiology of influenza A viruses such as pigs, to studies of transmissibility between different mammalian species or between individuals. Here, a team with extensive experience in the study of influenza A viruses and with abundant means to perform experimental studies in high biosafety laboratories, experimentally demonstrates several characteristics of one of the viruses isolated from a dairy cow. His results show that transmission of this particular virus between ferrets, a species commonly used as a model for humans, is inefficient. However, it highlights other characteristics that reflect the risk posed by these viruses, such as the ability to bind to both avian and human respiratory epithelial receptors.
Overall, it reflects, like many other papers, particularities of the virus that may constitute a high risk if other adaptive changes are added that may allow the virus to easily transmit and infect mammals, including humans.
Amie J. Eisfeld et al.
- Research article
- Peer reviewed
- Experimental study
- Animals



