Reactions to clinical trial testing efficacy of new treatment for vitiligo
A double clinical trial has analyzed the efficacy of ruxolitinib cream in treating depigmentation associated with vitiligo. The results are published in the New England Journal of Medicine.

Vitiligo. Freepik
Puig - Reacción vitíligo (EN)
Lluís Puig
Director of the Dermatology Department of the Hospital de la Santa Creu i Sant Pau and professor at the Autonomous University of Barcelona
The clinical trial is of excellent quality; efficacy is demonstrated, but five patients must be treated for six months to achieve a repigmentation response of 75% or more in one patient. If treatment is continued for one year, every second patient responds. Relapse after a period of time cannot be ruled out (not analyzed in this trial), but has been observed in experimental models.
The adverse effects described are those to be expected - the acneiform reaction stands out in 6% of patients. The plasma concentrations detected - by absorption through the skin in the treated areas - are relatively low, but not negligible.
The main problem would be the price of the treatment; the sale price of the cream in the United States is 50 dollars per gram (like gold, approximately). Half a gram (see figure) can treat an area equivalent to the surface area of two hands.
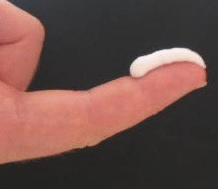
A patient with vitiligo on the face would have to apply 0.5 g twice a day for 6 months, equivalent to 180 g; to improve 75% or more in one patient, five patients would have to be treated. That would be 5 x 9,000 €.
Segurado - Vitíligo (EN)
Gonzalo Segurado
Dermatologist specializing in vitiligo at the Ramón y Cajal Hospital in Madrid.
The TRuE-V1 and V2 study is a well-designed study with a good number of patients studying the efficacy of a group of drugs called JAK pathway inhibitors in vitiligo. These drugs selectively block the action of the defenses that destroy melanocytes in the skin with the consequent loss of skin color. The novelty of this treatment compared to previous treatments lies in the selectivity of the JAK pathway inhibitors, since the former also aim to block the action of the defenses against melanocytes, but in a less selective manner. I think it is an interesting and positive study in that it is a first step that opens the door to research into new drugs in the future that act in a similar way. However, unless it is marketed at a reasonable price, I think it is difficult for the product under study to be of widespread application in patients with vitiligo, as there is not much difference in efficacy with some of the treatments we already have available and the cost is likely to be significantly higher. However, its good safety profile and ease of treatment are positive aspects to consider.
Translated with www.DeepL.com/Translator (free version)
Rosamarin et al.
- Research article
- Peer reviewed
- Experimental study
- Randomized
- Clinical trial
- People
