The early development of mammalian embryos, including us humans, is relatively well understood up to the moment of implantation in the uterus. A one-cell embryo divides until it forms a blastocyst, a ball of cells with an internal cavity and an asymmetrically positioned cell bud that will later give rise to a foetus. Prior to implantation, this process can be reproduced in the laboratory and observed under the microscope.
But once the blastocyst is implanted in the muscular wall of the uterus, we cannot observe its post-implantation development, unless we use non-invasive imaging systems or histological studies. These few days are the most important in embryonic development—that’s when gastrulation takes place, when three types of cell lines (ectoderm, mesoderm and endoderm) appear to give rise to the whole embryo and later to the foetus—and we cannot see this process. This is not the case with fish embryos, which are located inside transparent eggs that allow us a perfect view of all the steps of embryonic development, including gastrulation and later organogenesis.
Once the blastocyst is implanted in the muscular wall of the uterus, we cannot observe its post-implantation development
Research laws that apply in most countries don't allow the culture of human embryos in the laboratory beyond two weeks, and gastrulation begins at three weeks in humans. These first few weeks of embryo development are essential to determine the success or failure of gestation. And because we cannot do research at this stage—either because we do not have access to the pregnant uterus or because we cannot extend the necessary period of culture in the lab—we still do not understand why many pregnancies fail at these early stages. Traditionally, we have used our knowledge of the development of embryos from other species (such as mice) to extrapolate what could happen in human embryos. But Kathy Niakan showed us in 2017—using CRISPR gene editing tools to inactivate one gene, OCT4, in both human and mouse embryos—that the consequences of that gene’s inactivation were diametrically opposed in the two organisms, disproving the supposed equivalence between mice and humans in this area.
Previous studies with mouse and macaque cells
One possible solution emerged from pioneering experiments reported in August last year by researchers Jacob Hanna and Magdalena Zernicka-Goetz. The two laboratories independently mixed various types of pluripotent embryonic stem cells from mice in a culture medium, with a device that carefully moved them until, in rare instances, they spontaneously organised themselves into multicellular structures resembling a mouse embryo that continued to develop until a post-implantation stage—albeit in the laboratory, in a culture dish, outside the womb.
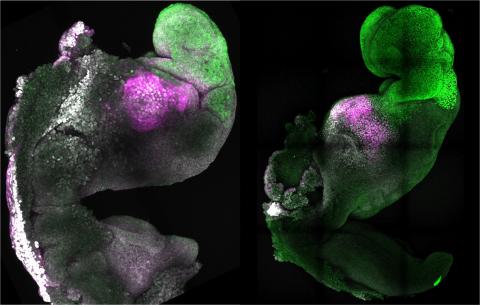
These mouse embryos, made and grown in the lab, looked remarkably similar to natural embryos and were named synthetic embryos
These results obviously piqued the interest of the scientific community, as they showed the ability to reproduce post-implantation stages of mammalian embryo development in the laboratory, without starting from a natural embryo (the product of fertilisation of an egg by a sperm). This had not been possible until then. These mouse embryos, made and grown in the lab, looked remarkably similar to natural embryos and were named synthetic embryos. They foreshadowed the ethical dilemmas that would arise when the same experiments were carried out on human embryos.
In April this year, a group of Chinese scientists led by researcher Zhen Liu succeeded in getting synthetic macaque embryos to tentatively begin gestation when implanted in the uterus of recipient females, but they quickly degenerated. In the laboratory, like what had been observed in mice, macaque embryos also managed to reach the important phase of gastrulation.
Competition between laboratories for human embryos
This week, we heard from two different news items that researchers had obtained synthetic human embryos in the laboratory, following protocols similar to those already described in mice and macaques. Firstly, according to British newspaper The Guardian, researcher Magdalena Zernicka-Goetz announced at a conference in Boston (USA) that she had obtained these embryos. The news did not come to our attention directly, but through comments to the newspaper and, initially, it was not yet backed by any scientific publication or preprint manuscript. Zernicka-Goetz subsequently indicated on Twitter that the research is pending publication in a scientific journal and that it involves embryo models—embryoids—rather than synthetic embryos, and that the embryoids’ purpose "is not to create life but to save it". Finally, a manuscript has also been deposited on bioRxiv, a preprint server, explaining that human embryos have been obtained from genetically modified pluripotent stem cells that show characteristics of natural embryos immediately after the implantation phase.
Palestinian researcher Jacob Hanna's response was swift. Less than 24 hours after publication of the Guardian’s article, his lab deposited a manuscript on bioRxiv with results showing that his team indeed succeeded in obtaining synthetic human embryos from pluripotent stem cells and that these laboratory embryos closely resemble natural human embryos at around 14 days of development.
This latest episode of competition between research groups working on the same topic, a very common situation in science, should not distract us from the actual achievement: synthetic human embryos
This latest episode of competition between research groups working on the same topic, a very common situation in science, should not distract us from the actual achievement: synthetic human embryos, in the laboratory, made from stem cells, up to a post-implantation stage. That is, embryos on which it is now possible to investigate all those biological questions about the initial phases of embryonic development that researchers could not access so far.
What protection do we give to synthetic embryos?
Now, we must decide what status we will grant to these synthetic embryos: will we grant them the same protection as natural embryos, or will we consider them a cellular grouping that resembles an embryo without being an embryo? Biology should give us the answer. We should know in detail how much these synthetic human embryos resemble their natural counterparts, genetically, cellularly, morphologically and in terms of the activation of various differentiation programmes.
We should know in detail how much these synthetic human embryos resemble their natural counterparts
If we are not able to see significant differences, I would have no argument against considering synthetic human embryos ethically as equivalent to natural ones. If there are differences between the two, and if it is clear that these synthetic embryos could not progress and give rise to babies if implanted, then we should consider them like we do organoids or any other grouping of human cells in culture.
In Spain, research on natural human embryos is strictly regulated, and limited to 14 days of culture in the laboratory. Research with natural human embryos (surplus pre-embryos from assisted reproduction technologies) is protected by various regulations (Law 14/2006, of 26 May, on assisted human reproduction techniques, and Law 14/2007, of 3 July, on biomedical research). It requires making a convincing case for the potential benefits of the scientific project to be carried out, the written consent of the couple or the mother, carrying out the experiments in authorised centres and by competent personnel, and the authorisation of the health authorities following a favourable report from the National Commission on Assisted Human Reproduction, which reports to the Ministry of Health.
However, all of the above rules apply to natural human embryos and, for the moment, do not apply to these new synthetic human embryos. Once again, science is leaping forward and testing the limits of the laws, posing new ethical challenges for us to solve. We will have to integrate these new technological advances into our project review procedures, both from a scientific and from an ethical point of view. Much work remains to be done, at all levels: scientific, legislative and ethical.