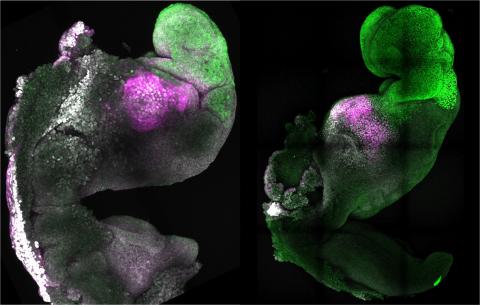
Reactions: macaque embryo-like structures developed and implanted in utero from stem cells
Chinese researchers have succeeded in developing macaque embryo-like structures from embryonic stem cells. They have also managed to implant them in the uterus of female macaque monkeys and develop a hormonal response similar to that of a gestation, although they have only survived for about a week. According to the authors, whose research is published in Cell Stem Cell, these models could be used to improve our understanding of embryonic development and to investigate the causes of some early miscarriages.

Female macaque and her cub in Djuanda Forest Park, West Java (Indonesia). Author: Rossche-Wikipedia.
Alfonso - Macacos (EN)
Alfonso Martínez Arias
ICREA research professor senior and researcher in Bioengineering Systems-MELIS at Pompeu Fabra University
The study is a step in the characterisation of embryonic development models based on embryonic stem cells. This work is based on the pioneering studies of Nicolas Rivron, who was the first to construct blastocysts with stem cells. The blastocyst is the first milestone in the development of a mammal and, of course, a human being. Rivron was able to build structures similar to mouse and human blastocysts, which he called 'blastoids'.
From a structural point of view (genes that express their cells), blastoids are almost identical to their natural counterparts, blastocysts. However, the proof of what they are is functional. What the blastocyst does is to implant itself in the uterus and, once well established, begin the construction of the organism with the process of gastrulation: the generation of the seeds of each tissue and organ as they arrange themselves in space. Until now, there was no evidence that blastoids could successfully implant and initiate embryo development. In the case of humans, for obvious ethical reasons.
In this paper, a group of Chinese researchers present data from macaque blastoids that appear to implant and, they say, initiate gastrulation in the maternal womb. The data appear to be of good quality, and although the frequency of success is low, the demonstration that these blastoids implant seems certain. Less clear is whether they gastrulate [the process by which the embryo acquires three germ layers]. My impression is that the gastrulation process is flawed. The few embryos that initiate this crucial phase of development collapse soon after the process begins.
The work is a further step in the characterisation of these embryonic stem cell-based developmental models, and there will be others. Their value will depend on two things: reproducibility and demonstration of their scientific value in providing new knowledge. The latter is, for the moment, a long way off. Macaques and humans are different, even in the way blastocysts implant. I see this work as a proof of concept that will be challenging to reproduce because of the cost of the research - working with primates is neither easy nor affordable - but it is clearly evidence that blastocysts will be, are, a useful tool for studying the early steps of uterine implantation, which affect many aspects of infertility.
Montoliu- Macacos (EN)
Lluís Montoliu
Research professor at the National Biotechnology Centre (CNB-CSIC) and at the CIBERER-ISCIII
Human pluripotent embryonic stem cells (hESCs) were first isolated from blastocysts in 1998 by J. Thompson's team. However, this breakthrough came 17 years after the same cells had been independently isolated from mouse blastocysts (mESC) by Martin Evans and Gail Martin. The biology of the pioneering mESC cells took the lead and has developed much further than that of hESCs.
With mESC cells, it is possible to regenerate an entire mouse by reintroducing them into another blastocyst or by aggregating them with preimplantation embryos from earlier stages. This has been used to generate the thousands of mutant mice that exist today, many of them animal models of human disease. mESCs can also be used to derive any other cell type in vitro, in the laboratory. And there have even been reports of the spontaneous, albeit very inefficient, emergence of synthetic mouse embryos in the laboratory by mixing these mESCs with other types of stem cells.
All this amazing versatility and malleability shown by mESCs cannot be replicated with hESCs, for a variety of scientific, legal and ethical reasons. For example, it is forbidden in most countries, such as Spain, to culture human embryos beyond 14 days.
For all these reasons, the researchers in this study, now published in the journal Cell Stem Cell, have opted to transfer the new culture and aggregation techniques developed in mouse mESC cells to cyESC, which are pluripotent embryonic stem cells from macaque (Cynomolgus), a non-human primate, evolutionarily related to us, as an intermediate approach to the experiment with hESC, which is currently unfeasible. And, indeed, they managed to obtain a macaque blastoid (similar but not identical to the blastocyst) from cyESC and, using these blastoids, they managed to differentiate different cell types in the laboratory, they managed to obtain embryos with the three germ layers and, after implanting them in the uterus of female macaques, they obtained evidence of gestation.
The study does not report the birth of any macaque babies so far. This is an experiment that cannot be performed in humans, due to the technical, legal and ethical limitations associated with it, but studies like this one, carried out in macaques, are gradually bringing us closer to such a possibility. The birth of non-human primates derived entirely from embryonic pluripotent stem cells grown in the laboratory (the next work that the authors of this study will no doubt attempt to carry out), without the need to go through fertilisation of an egg by a spermatozoon, as has been possible for years in mice, is getting closer and closer.
It remains to be seen how many of these advances will be tested on human embryonic pluripotent stem cells. And perhaps it would also be legitimate to ask whether we should undertake such experiments, how we should regulate them and for what purposes we should allow them to be undertaken.
Urries - Macacos (EN)
Antonio Urries
Director of the Assisted Reproduction Unit at the Quirónsalud Hospital in Zaragoza and member of the Quirónsalud Scientific Committee
The first thing to note about the article is that what they have generated are not embryos per se, but "embryoid" structures capable of behaving like an embryo in its first days of development.
In this work, Li's team manages to generate blastoids from monkey embryonic stem cells, with similar characteristics to natural blastocysts, managing to maintain their long-term development and even their implantation in the uterus of surrogate macaque mothers and generating pregnancies with the presence of early gestational sacs.
Although it is true that this type of embryoid structures have already been successfully generated in humans with morphology and structures similar to natural embryos, their culture beyond day 14 and their implantation in a woman's uterus is not permitted due to ethical issues. Therefore, being able to carry out this type of research in a species as closely related to ours as the macaque monkeys is an ideal model for the detailed study of the early stages of development of such vital mammalian organs as the heart, brain and neural tube. The beginning of organogenesis.
On the other hand, it can help us to delve deeper into certain implantation mechanisms and understand why pregnancies fail, detecting those anomalies that can lead to miscarriages. It can also guide us in the development of "synthetic" organs and tissues for transplants and help us understand the origin of certain diseases.
Naturally, it has the limitations of being a technique in a very preliminary phase, the result of a very complicated and inefficient process (around 25%), but with great potential for the future and a very promising applicability in humans.
Li et al.
- Research article
- Peer reviewed
- Experimental study
- Animals