Reactions to the generation of synthetic stem cell-derived mouse embryos
Research published in Nature shows the creation of synthetic mouse embryos derived from stem cells. The embryo model copies the stages of natural rodent embryo development that take place up to day 8.5 after fertilisation and includes regions of the brain, a neural tube and a structure similar to a beating heart.
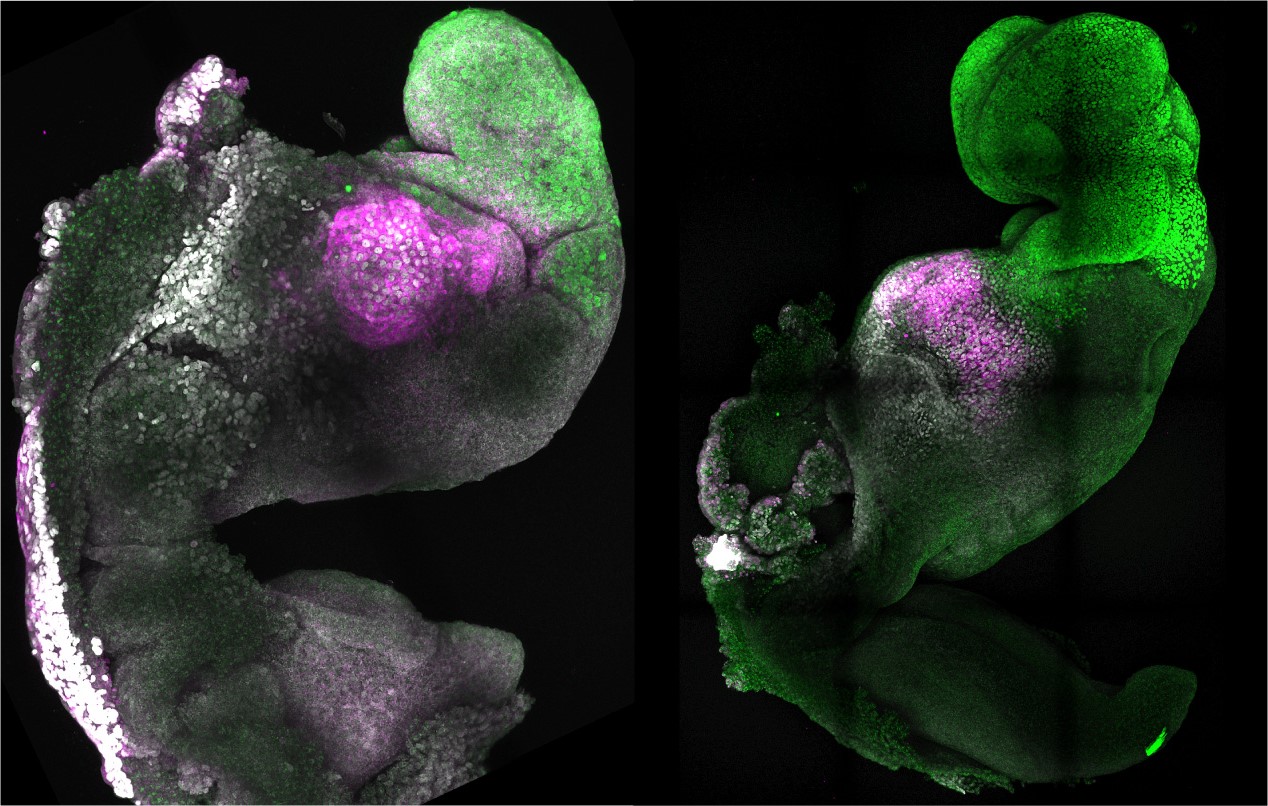
Natural and synthetic embryos show comparable brain and heart formations side by side. Authors: Amadei and Handford.
Alfonso Martínez - embrión ratón EN
Alfonso Martínez Arias
ICREA research professor senior and researcher in Bioengineering Systems-MELIS at Pompeu Fabra University
This work is an important addition to the work published by Jacob Hanna's group in Cell a few days ago. Both represent a major breakthrough, proving the need for interaction between embryonic and extra-embryonic cells in the formation of a mammal. Moreover, they show that it is possible to initiate mammalian development outside the womb. But it is more a 'proof of concept' than an absolute achievement. As in the case of Hanna's work, the number of synthetic embryos obtained with the method is small (in the order of 1% of the initial cultures) and their development collapses prematurely after a few days in culture. It is also important to note that the structures obtained, although they contain the elements of an embryo, have significant deficiencies and most of these structures are damaged or incomplete. It is also unclear what, exactly, is intended to be obtained from these structures, which, one has the impression, emerge from the culture almost magically.
The need to emphasise the defects and underperformance of the experiment is important, because the idea that a mouse embryo has been obtained in culture will give rise to the notion that the same will soon be done with human cells and that a human embryo will be obtained. It is very likely that this will happen in the future and one value of this experiment is to alert us to this possibility so that we consider the ethical aspects of such experiments and the social impact they may have.
Of course, both papers are important, but they are at very early stages and we will have to wait and see how they develop. Much more important will be to see what applications the system has. To take that step, efficiency and accuracy will have to increase. At the moment, female mice make perfect embryos more efficiently.
"My group has developed an alternative system to study mammalian development from stem (gastruloid) cells, but it is not a competing system".
Lluís Montoliu - embriones ratones EN
Lluís Montoliu
Research professor at the National Biotechnology Centre (CNB-CSIC) and at the CIBERER-ISCIII
The fundamental challenge in biology remains to understand how a living organism as complex as any of us develops from a single cell. Understanding how hundreds of different cell types emerge during embryonic development from a single single cell embryo, which is the product of the fertilisation of an egg by a sperm. To answer these questions, we can witness the development of other animals, such as zebrafish, whose embryos develop externally, inside transparent eggs that allow us to see everything that is going on inside. However, for mammals, like us, it is much more difficult, since the embryo implants in the uterus of the female and develops inside anatomical structures that are difficult to see and access. Our ability to observe in detail, directly, what happens during the development of a mammalian embryo is limited to the early stages, prior to the implantation of the embryo in the uterus.
This is the challenge that Magdalena Zernicka-Goetz's lab set itself and whose results she now presents in her paper published in the journal Nature. She has managed to reproduce the initial stages of development of a mammalian embryo, from a mouse, in the laboratory, without needing the participation of a female to implant the embryo. And they have also achieved this without the need to resort to the fertilisation of an egg by a spermatozoon. Instead, these researchers have used different types of embryonic stem cells (stem cells) which, when mixed together, give rise to a new biological structure that looks very much like a natural embryo, without being one. These are synthetic embryos, developed entirely in the laboratory.
For the extra-uterine development of these synthetic embryos, Zernicka's laboratory at Caltech (California, USA) has used a device, an artificial incubator, which makes it possible to simulate the physiological conditions that exist in the female uterus. This ingenious technical solution was developed by the laboratory of Jacob Hanna of the Weizmann Institute in Israel, co-author of this study and who also reported similar experiments a few weeks ago, published in the journal Cell.
The synthetic embryos reach a stage equivalent to that of natural embryos at 8-9 days gestation, almost half the pregnancy time in mice, which is 19-20 days. And they manage to develop very similar anatomical structures, such as the heart, with its beat, and the brain, with its different areas. These synthetic embryos are not embryos, but they are useful for research.
Since these synthetic embryos are derived from embryonic stem cells in culture, they can also be generated from cells that contain a mutation in some gene, and thus investigate the effect that this mutation produces in the initial phases of development, observing directly in the laboratory what happens at each moment. This is a privilege that researchers did not have before with mammalian embryos.
We are undoubtedly facing a new technological revolution, still very inefficient (it is very difficult to get stem cells to spontaneously generate a synthetic embryo), but with enormous potential. It is reminiscent of such spectacular scientific advances as the birth of Dolly the sheep, which we met in 1997, reconstructing an embryo with the nucleus of a somatic cell, or the inducible pluripotent embryonic stem cells, iPS cells, described by Yamanaka in 2006, which led him to win the Nobel Prize in Physiology or Medicine in 2012, shared with John Gurdon, pioneer of animal cloning in amphibians. A revolution that naturally also raises new ethical dilemmas, if we ever think of transferring these experiments to the human species for the generation of synthetic human embryos, perhaps with the aim of using them to obtain new tissues or organs to repair or replace those that are damaged, as Hanna has already proposed to explore, through a company he has created ad hoc.
Ginaluca Amadel et al.
- Research article
- Peer reviewed
- Animals