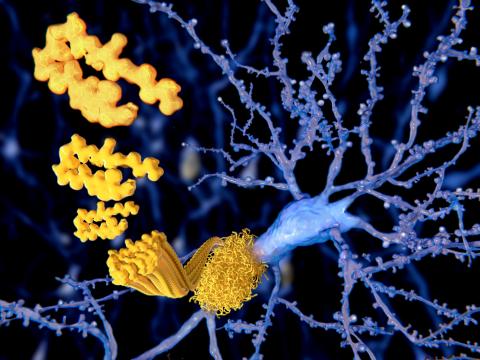
Reaction: study shows Alzheimer's drug lecanemab helps neutralise the effects of small toxic amyloid aggregates
Research led by scientists at Harvard Medical School (USA) has isolated small aggregates of amyloid from the brains of post-mortem Alzheimer's patients. The achievement has made it possible to study the structure of these "clumps", which exist outside plaques and are considered highly toxic, and to test their effect on synapses. In addition, the authors have shown that the drug lecanemab, recently approved by the FDA, is able to bind to them and help neutralise their action. The results are published in the journal Neuron.
Xavier Morató - lecanemab EN
Xavier Morató
Director of Clinical Trials at Ace Alzheimer Center Barcelona
The mechanism of action of lecanemab is well studied in vitro, but more studies are needed to understand which specific species of b-amyloid peptide the monoclonal antibody binds to in order to design the famous starting/stopping rules. That is, to be able to help neurologists make decisions about when to start and stop treatment (should we continue treatment once we have reduced/eliminated the amyloid plaque in the brain). Based on the results of this work, continued administration of lecanemab in patients with Alzheimer's disease, once amyloid plaque levels have been reduced, may help to reduce the neurotoxicity of oligomers and soluble fibres at the synapse.
Each of the three monoclonal antibodies that have shown positive results in phase III clinical trials (aducanumab, lecanemab and donanemab) have different affinity for these aggregated amyloid peptide species. We therefore need to better understand the differences between them in order to use them as efficiently as possible.
The study has no major limitations to consider.
Stern et al.
- Research article
- Peer reviewed
- Experimental study
- People