University of Navarra
If you are the contact person for this centre and you wish to make any changes, please contact us.
PhD in Pharmacy, Professor of Human Physiology at the University of Navarra, member of the CIBER Physiopathology of Obesity, Carlos III Health Institute and IDISNA (Navarra)
Researcher in Architecture at the University of Navarra
Researcher in the Gene Therapy and Regulation of Gene Expression Programme and Director of Innovation and Transfer at Cima University of Navarra
Professor of Immunology at the University of Navarra, CIMA researcher and co-director of the Department of Immunology and Immunotherapy at the Clínica Universidad de Navarra.
Assistant Professor at the Department of Physics and Applied Mathematics of the Faculty of Science.
Researcher in the Mind-Brain Group of the Institute for Culture and Society (ICS) at the University of Navarra
Scientific director of the International Center for Neuroscience and Ethics (CINET), Tatiana Foundation
Senior Researcher of the Gene Therapy in Neurodegenerative Diseases Programme at the Centre for Applied Medical Research (CIMA), University of Navarra
Co-coordinator of the working group on Nutrition of the Spanish Society of Epidemiology (SEE), Professor of Preventive Medicine and Public Health at the University of Navarra, and member of CIBERobn
Professor of Preventive Medicine and Public Health at the University of Navarra and Adjunct Professor in the Department of Nutrition at the Harvard T.H. Chan School of Public Health (United States)
Professor and Head of the Preventive Medicine and Public Health Department

A meta-analysis that included 45 studies involving more than 330,000 people has analysed the association between the time spent exposed to digital screens and the risk of developing myopia. The results of the study, published in JAMA Network Open, show that the risk of myopia increased significantly by just over 20% for every hour of daily use after the first hour.

Thousands of people congregate at the San Fermín festival in Pamplona. By analysing camera images from the Plaza Consistorial during the chupinazo in four editions, a team has modelled what the movement of this human tide looks like. The physical theory of dense crowds can be applied in other circumstances, say the authors, who include scientists from the University of Navarra. In their paper published in Nature, they offer a strategy to anticipate these movements in real time and help prevent events such as avalanches.

In a group of people at high cardiovascular risk, low to moderate wine consumption was associated with fewer cardiovascular events (cardiovascular death, myocardial infarction, stroke or heart failure), according to a study. The analysis uses urinary concentrations of tartaric acid, a substance found in grapes and grape derivatives, as a biomarker of wine consumption. It finds that consuming between three and 35 glasses per month was associated with fewer cardiovascular events than in people who consumed fewer than three or more than 35 glasses. The study, published in the European Heart Journal, included more than 1,200 participants from Spain's PREDIMED study with an average age of 68 years.

According to a study published in Science, social media content containing misinformation provokes more moral outrage than content containing accurate information, and this outrage facilitates the spread of misinformation. In addition, the results also showed that people are more likely to share this outrage-provoking misinformation without reading it first.

The use of the antidepressants escitalopram, paroxetine, and duloxetine is associated with greater weight gain than the use of sertraline, according to the results of an analysis comparing data from more than 183,000 adults treated with one of eight types of antidepressants. Among these, bupropion is associated with the least weight gain, concludes the study, which is published in Annals of Internal Medicine.

People who take multivitamins daily do not have a lower risk of mortality than people who do not use these products, according to a meta-analysis published in JAMA Network Open. The study pooled data from more than 390,000 people, followed for about 20 years in three different cohorts in the United States.
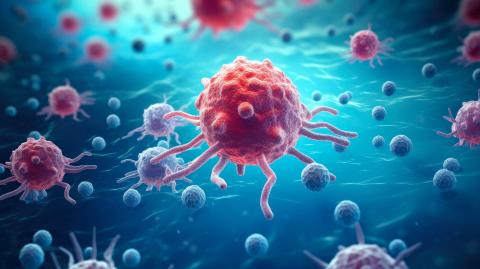
CAR-T cell therapies may, in some cases, produce tumours secondary to treatment. A few months ago, the US Food and Drug Administration (FDA) said it was assessing this risk. Now, a study conducted at Stanford University Medical Center (USA) has tracked 724 patients who received this type of treatment since 2016. Of these, 14 developed another blood tumour, but only one was a T-cell lymphoma that could be a direct consequence of the therapy. Further analysis ruled out this link. The results are published in the journal NEJM.

People who eat more ultra-processed foods have a "slightly higher" mortality rate, according to an analysis published in The BMJ. The study analysed data from more than 110,000 people followed up for over 30 years in the United States. The correlation between ultra-processed food intake and all-cause mortality was strongest for the meat, poultry and seafood group.

A monoclonal antibody called prasinezumab reduces the worsening of motor symptoms in people with Parkinson's disease who have rapidly progressive disease, according to an analysis of a phase 2 clinical trial published in Nature Medicine. These findings suggest that clinical efficacy of prasinezumab, which works by binding to alpha-synuclein protein aggregates, is seen after one year of treatment in such patients. According to the authors, more research is needed to determine whether the antibody can be effective in people with slower disease progression after longer periods of treatment.

A phase 2 clinical trial in France has examined whether taking an oral anti-diabetic drug called lixisenatide - a GLP1 receptor analogue, similar to those also used for weight loss - also has an effect on the progression of Parkinson's disease. The results indicate that there is a modest but significant decrease in the progression of motor symptoms of the disease, although side effects were also observed. The results are published in the journal NEJM.