Reaction: New fusion protein tested for glioblastoma treatment
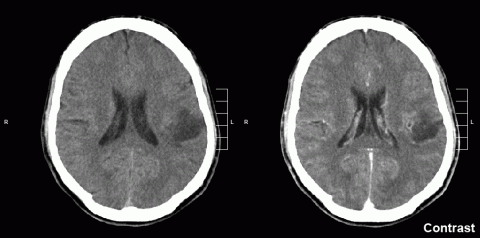
A team of scientists led by the University Hospital Zurich (Switzerland) has tested a new treatment for glioblastoma, a highly aggressive nervous system tumour with a poor prognosis. The therapy consists of a fusion protein that combines the TNF factor - a key factor in the processes of inflammation and immune response - with an antibody that targets the tumour matrix. The researchers, whose results are published in the journal Science Translational Medicine, have studied its action together with a type of chemotherapy both in mice and in six patients included in a phase 1 clinical trial.
Ignacio Melero - proteína fusión glioblastoma EN
Ignacio Melero
Professor of Immunology at the University of Navarra, CIMA researcher and co-director of the Department of Immunology and Immunotherapy at the Clínica Universidad de Navarra.
The article has two parts. Most importantly, the authors have launched a phase 1 clinical trial with an immunocytokine resulting from the fusion of an anti-fibronectin antibody (L19) with the inflammatory mediator TNF. The treatment, which is administered together with chemotherapy and appears tolerable, has been tested in six patients with glioblastoma who have progressed refractory to conventional treatment. This is a very limited number of patients, but with surprisingly long follow-up (they often die earlier), and in three cases the disease has significantly reduced in size.
Hopefully these results will be confirmed in the remaining series of patients, which is still very short but extremely promising.
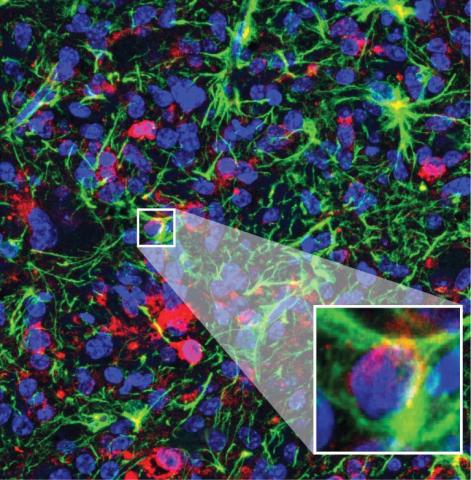
The first part of the article deals with experimental work in mice carrying intracranial models of glioblastoma. The experimental treatment consists of an immunocytokine, consisting of an antibody targeting the extracellular matrix of tumours (rich in fibronectin) and the inflammatory mediator TNF. They observe a relatively discrete anti-tumour effect in the models and analyse the mechanisms of action, which have to do with enhancing inflammation and antigenic presentation in the tumour. The actual resemblance of these intracranial tumour grafts to glioblastoma in humans is questionable, but the mechanisms revealed are of interest and have led to the above-mentioned clinical trial, the first results of which are very promising.
Sarah-Jeanne Royer et al.
- Research article
- Peer reviewed
- Clinical trial
- People
- Animals
- Experimental study