What vaccine is used?
A third generation vaccine is being used, marketed in Europe as Imvanex and in the United States as Jynneos. Both are manufactured by Bavarian Nordic Laboratories and differ only in some minor aspects of preparation. Unlike the older ones, it is a vaccine with an attenuated smallpox-related virus that does not replicate and does not cause disease, and is therefore considered safer. Although already approved for use against smallpox, the European Medicines Agency (EMA) has just recommended extending its use to monkeypox.
How effective is it?
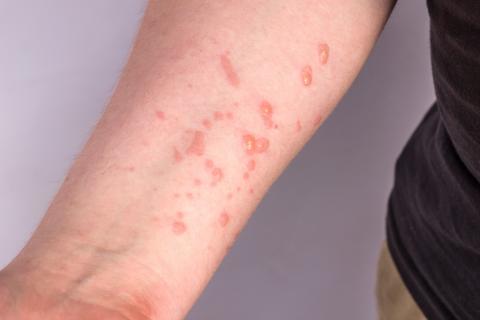
Efficacy studies in humans have not yet been conducted with the Imvanex vaccine. However, animal studies show a similar response and efficacy to older vaccines. Although not developed specifically against monkeypox, but against smallpox, the similarities between the viruses allow immunity to be generated against both. According to an analysis during an outbreak in Zaire in the 1980s, the effectiveness of previous vaccines in protecting against monkeypox is about 85%.
Who can receive it?
According to the Spanish Society of Epidemiology, vaccination against monkeypox is not recommended for the general population, as the risk is considered low. At present, the latest update approved by the Public Health Commission on 12 July recommends and prioritises vaccination in different groups of people depending on whether it is pre-exposure prophylaxis (if there has been no known contact with an infected person) or post-exposure prophylaxis (if there has been contact).
Pre-exposure prophylaxis:
—In people under 45 years of age who engage in high-risk sexual practices, primarily but not exclusively gay, bisexual and men who have sex with men who have not previously passed the disease or received the smallpox vaccine.
— In people at occupational risk such as healthcare workers in specialised practices caring for people with high-risk practices, laboratory staff handling potentially contaminated specimens, or staff involved in surface disinfection in specific premises attended by people with multiple partners.
Post-exposure prophylaxis:
—In close contacts of confirmed cases who are at high risk of severe disease (infant population, pregnant women and immunocompromised persons), as well as healthcare and laboratory personnel with contact with confirmed cases and who have had any incidence of PPE (Personal Protective Equipment) use.
Why is priority given to people under 45 years of age?
In Spain, vaccination against smallpox was compulsory until 1979 and was administered at 20 months of age. At that time it was removed from the vaccination schedules, as the disease was considered eradicated. It is assumed that people who have been vaccinated maintain a certain degree of protection against monkeypox as well.
Is it safe?
The safety profile of the vaccine is favourable, with the possibility of mild to moderate side effects, according to the EMA. The Spanish Society of Epidemiology has issued a statement in which it considers that the vaccine "is safe to use and its side effects are similar to those of other vaccines, and may cause headaches, nausea, muscle pain, asthenia (tiredness) or pain in the area of the puncture".
Is it approved for all population groups?
In principle, the vaccine is not authorised for use in children, pregnant women and breastfeeding women. However, as stated in the latest update from the Public Health Commission, it is not contraindicated in these populations and has been used in small outbreaks in the UK in 2018 and 2019 with no adverse effects reported. It is recommended for use after individualised assessment in children of any age and pregnant women in any trimester of pregnancy, as the disease may be more severe and cause sequelae in these populations.
In whom is it contraindicated?
In people with hypersensitivity to the active ingredient or to any of the residues of which it contains traces: chicken protein, benzonase, or the antibiotics gentamicin and ciprofloxacin.
How many doses are given?
The standard schedule is two doses administered at least 28 days apart. However, due to the shortage of available vaccines, at present only one dose will be administered. In the case of post-exposure prophylaxis, one dose will be given within 4 days of close contact, although it may be offered up to 14 days, in which case it would not be so much to prevent as to attenuate possible disease. In immunocompromised persons, two doses should be administered.
These recommendations will be adapted according to the epidemiological situation and the availability of vaccines.
How many doses are available?
There is currently a global shortage of vaccines, which are manufactured by a single laboratory. Spain purchased 200 doses of Imvanex in June by direct purchase from the Netherlands. Subsequently, the European Commission has made a joint purchase of just over 160,000 doses of Jynneos vaccine from the United States, where it was already approved against monkeypox as of 2019. Spain, which has already received 5,300 doses, will initially receive 10%. Further shipments are planned for the next two months. In addition, Spain has two million doses of an older, second-generation vaccine in stock. However, its use is not recommended in the current situation.