Reactions to study finding a neuron migration pathway active up to two years of age
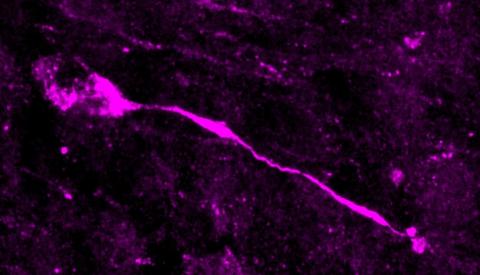
A study published in Nature in which researchers from the University of Valencia have participated has identified a neuron migration route that begins in the foetus around mid-gestation and continues until between two and three years of age. The route extends from the lateral ventricle, where these cells are born, to the entorhinal cortex, an area related to the regions where memory and learning are consolidated. There, neurons await signals that induce them to mature, providing plasticity to the brain after birth.
Aixa - Neuronas (EN)
Aixa Morales (EN)
Researcher at the Instituto Cajal, CSIC
The work comes from an excellent group such as Arturo Álvarez-Buylla, one of the most prominent scientists in the field of nervous system development, especially human brain development. He received the Prince of Asturias Award in 2011. The other senior author, Shaun Sorrells, a former postdoctoral researcher in Álvarez-Buylla's group, leads a young group. The study is excellent for its in-depth knowledge of the anatomy of the human brain at perinatal stages, the use of state-of-the-art techniques such as massive single-cell sequencing and the use of numerous cell population markers in immunohistochemistry.
The authors have been studying the migration of immature progenitors and neurons in the human newborn and infant brain for years. Thus, they had published the existence of different streams of immature progenitors and inhibitory neurons in perinatal stages from the subventricular zone of the lateral ventricles to the olfactory bulb and medial prefrontal cortex (Sanai et al., 2011) and to the frontal lobe of the cortex (Paredes et al., 2016). In addition, they had also shown that there are progenitors and immature excitatory neurons in the paralimbic region of the amygdala of the newborn and young children and that at least the immature neurons persist until late in life and mature progressively (Sorrells et al., 2019).
Now they have found immature inhibitory neurons in the entorhinal cortex in children's brains, at least until the age of 2-3 years (Nascimiento et al., 2024). The presence of these progenitors and immature neurons in various locations in the brains of infants and young children indicates that there is greater plasticity than was thought a few years ago, as the process of migration and maturation of these neurons may well be conditioned by external stimuli and experiences (as determined in animal models).
The entorhinal cortex region is involved in the hippocampal circuitry involved in memory (spatial and episodic) and learning and is one of the first brain regions affected in Alzheimer's disease. The authors speculate that perhaps the fact that the development of these immature neurons in the entorhinal cortex is so delayed in time makes them more susceptible to neurodegeneration in Alzheimer's disease. However, we still know very little about how this disease is triggered, so it is difficult to draw conclusions linking these early neuronal populations in childhood and a neurodegenerative disease mostly associated with ageing.
Although there is controversy about their existence, populations of neural stem cells and immature neurons in the human hippocampal dentate gyrus are altered in Alzheimer's disease (Moreno-Jiménez et al., 2019; Terreros-Roncal et al., 2021).
All studies performed on human brains are limited in that they are purely descriptive and we cannot perform any cause-effect analysis. We depend on the advancement of human cell models such as cerebroids or the use of animals such as mice or non-primate monkeys.
I declare that I have no conflict of interest with the publication under review or any of the publications referred to, as I have not participated in any of the articles.
Pla - Neuronas (EN)
Ramón Pla Ferriz
Associate Professor in the Department of Human Anatomy and Psychobiology at the University of Murcia
Multiple cell migrations, both neuronal and glial, occur during embryonic development. Most of them end perinatally, although some continue into adulthood. In some cases there are cells that remain in an immature stage, acting as a cellular reservoir with or without the capacity to divide.
This is what is shown in the article by Alderman et al. so that these cells are then able to disperse and occupy adjacent tissues. These migrations, first discovered mostly in mice or other laboratory animals, are being corroborated in recent years in humans, as shown in the article by Nascimento et al. (2024). The process of cell migration allows different cell populations in the developing brain to occupy different sites from where they were born. This ensures that there is high neuronal diversity at a particular site, even if the sites of production are discrete and far apart. Many of the migrations that have been discovered originate in the ventricular zones (part of the tissue lining the lumen of the neural tube) of the ganglionic eminences, which lie beneath what will become the future cerebral cortex (Anderson et al. 2001, Flames et al. 2004, Nóbrega-Pereira et al. 2010, Pleasure et al. 2000, Valiente et al. 2009).
These migrations include rostral migration (RMS), whose cells leave up to the olfactory bulb ((Lois et al. 1994), tangential migration, lateral migration (LMS) (Carney et al. 2006), medial migration (MMS) (Touzot et al. 2016) or caudal (CMS) (Touzot et al. 2016, Butt et al. 2005, Nery et al. 2010), etc. (Ruiz-Reig et al. 2017), which supply cells to the cerebral cortex through various pathways, to the future amygdala, hippocampus, etc.
One important thing to keep in mind is that not all migrating cells are neurons; there are also glial cells such as oligodendrocytes, which use the same mechanisms and pathways to move as neurons. It is important to keep these ideas in mind to put the article in context.
The article is part of a stream of papers that are confirming in humans the cell migrations that have already been reported in laboratory animals. In this line, the same research group has already published that RMS and LCS also occur in developing brains. Unlike in rodents, RMS in humans ends a few years after birth. This does not detract from the work presented here, because of the difficulty involved in working with human material. Furthermore, in my opinion, the work corroborates:
That the patterns of construction of our brains were established early in the evolutionary line. In fact, the topic at hand, neural migrations, is observed in animals ranging from sharks (cartilaginous fish) (Carrera et al. 2008) to, of course, various orders of mammals.
The importance of continuing with basic studies in animals, since the fact that we share with them the formation of our brain allows us greater manipulation and, therefore, a better and deeper understanding of the underlying mechanisms, which is difficult to achieve with human material.
The authors state that a new migration from the ventricular zone of the caudal eminence to the entorhinal cortex has been discovered. This migration is consistent with that already described by other authors in rodents, the CMS and LMS. Therefore, in my opinion, it has the novelty that was not known to exist in humans, although it was intuited by the data in other species. Thus, it is easy to see that in the coming years more articles will appear in this line, which does not detract from the work done here. What is not clear from the study is the type of cells that migrate, let alone the proportion of them. The authors identify them by markers as immature neurons, but the markers used are also expressed in oligodendrocytes at these ages and in these locations.
It would have been interesting to have more specific markers for neurons and oligodendrocytes to differentiate them and also to know in what proportion they migrate. It is quite clear from the gene expression analysis that both groups are in the migration pathway, so I assume that as in the animal studies, it will be a heterogeneous mixture of cells, although we do not know their proportion. The authors go so far as to conclude the percentage of 'neurons' in tangential and radial migration by the arrangement of their guidance process, but the lack of videomicroscopy studies leads me to say that it is very risky to make such a claim. Videomicroscopy time-lapse studies in animals have shown that cells do not follow a single direction during their migration, adopting multiple directions in their guidance process. Thus, it is easy to misjudge the mode of migration.
In conclusion: the article is obviously of good quality, but suffers from a few things, which may be due to the difficulty of working with human tissue (histology is not always straightforward in human tissue). The novelty of the article does not lie in the type of migration, as this was previously known in animals, but in the fact that it has now also been demonstrated in humans.
The authors state emphatically certain things that are not so clear. One of them, as explained above, is the type of migrating cells. They claim that they are immature neurons, but the lack of specific markers raises some doubts. Oligodendrocytes also migrate and it seems, from gene expression analysis, that they do too. But how many of each kind are migrating? That question remains open. The lack of experimental embryology experiments leaves us in doubt as to what the pattern of migration is, how long it takes, the speed, whether it is jumping or continuous, and so on.
The boldest they say is the implication it could have on Alzheimer's disease. One of the structures most affected in this disease is the hippocampus, which has direct connections to the entorhinal cortex. They claim that because the entorhinal cortex is affected in Alzheimer's patients, a problem in this migration could have consequences for Alzheimer's disease. Such conjecture is still hazardous, since, being two highly synaptically related structures, it could be that a mortality in the hippocampus could end up producing the same in the entorhinal cortex. This is the same as the chicken and the egg, which came first.
It is true that it is important and that defects in this migration could be present in this or other diseases. The entorhinal cortex receives multiple connections from both the limbic system and the cortex, and is therefore involved in a multitude of associative cognitive processes.
Nascimento et al.
- Research article
- Peer reviewed
- People