Reaction to the study about the use of antivirals in monkeypox patients
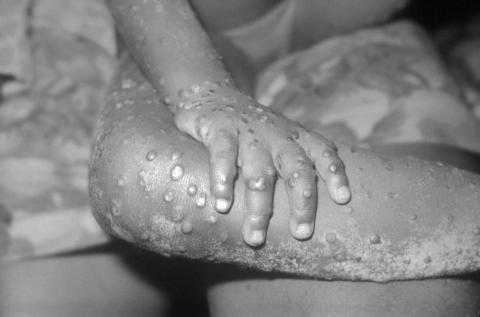
A study published in The Lancet Infectious Diseases analyses the response of seven monkeypox patients diagnosed in the UK between 2018-2021, treated with the antivirals brincidofovir and tecovirimat.
Monkeypox virus illustration. / Adobe Stock
Mar Faraco sobre uso de antivirales contra viruela del mono - EN
Mar Faraco
Former president and current secretary of the Association of Foreign Medical Doctors (AMSE) and head of the Servicio de Sanidad Exterior in Huelva
It is interesting as a description but provides little or nothing, because of the very limited number of cases covered. There is a brief general summary, no comparison, no associated laboratory data on viral detection nor general test data, etc.
Specifically, brincidofovir was given quite late and in addition to not seeming to have much effect (perhaps also because of the delay?) it altered the liver tests. But with three cases and without knowing the dose, as they themselves say, it is not assessable (perhaps it is useful as a small clue to follow if it is decided to reuse).
As for tecovirimat, approved in 2022 by the EMA (European Medicines Agency), with only one case treated there is nothing real to say, although it does not contradict the data for which it has been authorised, and it has had no side effects.