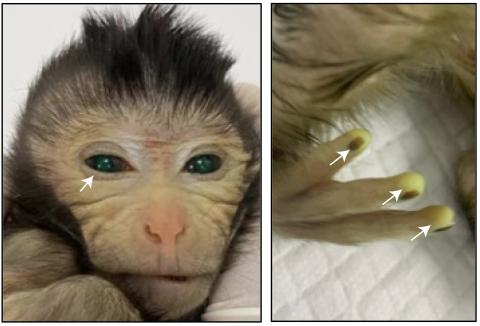
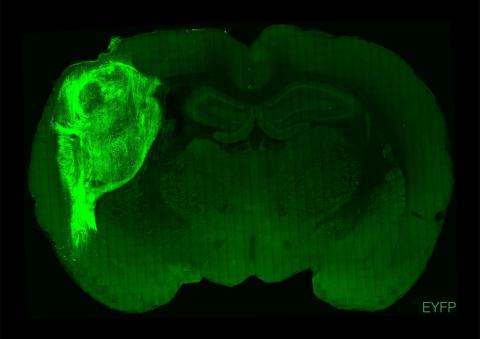
Reactions: scientists regenerate neural pathways in mice with rat cells
Two independent research teams have managed to regenerate brain circuits in mice using neurons cultivated from rat stem cells. Today, both studies were published in the journal Cell. The research, in which chimeras of different species were generated, delve into how brain tissue forms and present new opportunities for restoring lost brain function due to diseases and aging.
Lluis Montoliu - quimeras ratones EN
Lluís Montoliu
Research professor at the National Biotechnology Centre (CNB-CSIC) and at the CIBERER-ISCIII
In October 2022, Sergiu Pasca's lab from Stanford University, surprised the world by injecting neurons originated from human brain organoids (simplified mini-brains developed from stem cells in a culture medium) into a developing rat brain, using athymic newborn rats (and therefore unable to reject human cells). Those chimeric rat brains, with human neurons, grew normally as the animal continued to develop, and the human cells interconnected with the rat neurons to the extent of establishing functional somatosensory circuits. The authors demonstrated that excitation of the rat whiskers activated the human neurons, confirming that these cells were fully embedded and intercommunicated within the rat brain. Logically, this study raised ethical dilemmas associated with the creation of chimeras, limited originally to the brain, but in which human neurons and rat neurons were mixed. According to the authors, the potential use (and therefore the ethical justification) of this experiment was that it might offer a way to validate, functionally, the state of human neurons from both healthy people and, more importantly, from patients with neurological disorders. As, in these patients, it is usually very difficult to identify what may be wrong in their neuronal function. That paper, from less than two years ago, surely opened the door to the two papers being published today, in which two teams of scientists, working independently, demonstrate that rat stem cells can complement and restore genetically disabled brain regions in mouse embryos.
First, Jun Wu's lab, from the University of Texas Southwestern at Dallas, reports a surprising experiment in which he inactivates a gene (Hesx1) in mouse embryos using CRISPR gene-editing technology. This gene is necessary for forebrain development and, therefore, the mice derived from these embryos would lack the frontal brain region. However, at the blastocyst stage, the researchers inject the mice with pluripotent embryonic stem cells from rats, and these eventually supplement the neuronal deficit and replenish the mouse forebrain, which is now formed from rat neurons (from the injected stem cells). The head and brain of a rat is larger than that of a mouse. However, the rat neurons grow at a mouse pace and fill the space that is expected in a mouse head, just as if they were mouse neurons. The mice, as far as the researchers can confirm, behave normally.
The second experiment, developed by Kristin Baldwin's lab at the Scripps Institution in La Jolla and Columbia University in New York, has a similar structure. This time the researchers used two different strategies to eliminate or genetically silence (using a conditional mutation strategy with the Cre/loxP system) the mouse neurons that grow into the olfactory bulb, which is located in the most anterior part of the brain. And these mouse embryos without olfactory bulb or with an inactivated olfactory bulb (and therefore anosmic, unable to perceive any odor) are injected with pluripotent rat stem cells that end up colonizing or complementing with rat neurons the non-existent or non-functional olfactory bulb of the mouse, thus recovering the mice's olfactory capacity. This experiment demonstrates, beyond the previous one, that this complementation isn’t only structural (the rat neurons fill or complement the space or function that should be performed by the olfactory neurons of the mouse) but totally functional. Mice recover their sense of smell due to the rat’s olfactory neurons, even if the recovered sense of smell is not yet equivalent to that of the control mice.
These are undoubtedly very remarkable advances in neuroscience that allow many other complementation experiments to be tackled. It doesn’t seem like all regions of the developing brain of one species could be complemented with neurons from another species with the same level of success. However, the fact that two laboratories working independently reached similar conclusions is noteworthy. It endorses the robustness and credibility of their findings, and confirms the utility and applicability of this novel form of neuronal complementation via different species.
Additionally, since both teams use brain chimeras between mice and rats, the ethical issues associated with these experiments are much more limited than the ones that would arise if human cells were used instead. I applaud the decision of these two laboratories to do these complementation experiments using two rodent species, such as the rat and the mouse, which, from an evolutionary perspective, are separated by about 20 million years. The conclusions that result from this series of experiments in rodents enable the development of future applications in humans. With this research, they will already be approached with a basic understanding of the neural processes associated with these complementation experiments.
Rüdiger Behr - quimeras EN
Rüdiger Behr
Head of the Platform Degenerative Diseases at the German Primate Center (DPZ), Leibniz Institute for Primate Research, Germany
The publications by Huang et al. and Throesch et al. deal with the production and investigation of chimeras between mice and rats. Chimeras are organisms that consist of cells that originate from two different fertilization processes (embryos). A distinction must be made between intra- and interspecies chimeras. In intraspecies chimeras, the chimeric cells both belong to the same species. For example, mouse-mouse chimeras are used in the studies published here. In these new studies, however, interspecies chimeras between mouse and rat are also produced. In this case, the cells that form an organism originate from two different species. Interspecies chimeras in particular are of great interest from a developmental and evolutionary biology perspective. However, interspecies chimeras can also provide very valuable insights regarding the production of transplantable organs for human medicine. This is a high-priority research goal in view of the decades-long shortage of donor organs for transplantation in patients.
Chimeras must be clearly distinguished from hybrids. In contrast to chimeras, hybrids develop from just one fertilized egg cell. In hybrids, however, the male sperm and the female egg come from two different (but closely related) species. For example, the sperm of a donkey can successfully fertilize the egg of a horse. The resulting embryo develops into a mule. In a hybrid, all the individual cells of the organism itself are already a ‘mixture’ of the two parent species. In an interspecies chimera, on the other hand, each individual cell is clearly assigned to one species.
In the two current studies, the chimeras are generated experimentally at the so-called blastocyst stage. Around ten ‘all-rounder’ stem cells are injected into an embryo that is a few days old and around a tenth of a millimeter in size, which at this stage is a small fluid-filled bubble of cells. The injected stem cells integrate into the recipient embryo and co-develop with it more or less efficiently until birth. The integration of the injected stem cells is much more efficient if the recipient embryo is unable to form individual tissues or organs itself due to a specific genetic modification. These free anatomical niches in the developing embryo are then taken up and filled particularly efficiently by the injected chimeric cells. This process is known as blastocyst complementation.
Huang et al. can be credited with making the blastocyst complementation process much faster by combining the genetic methods for opening a niche into which the chimeric cells can efficiently implant with the methods of previous blastocyst complementation in one step. This new combined technology will greatly accelerate chimera research - especially in larger mammals with a longer generation time. Given that some of the authors of this study work at a Chinese primate center, it can be assumed that this technology will now also be used in non-human primates.”
“Throesch et al. were able to show for the first time that chimeric cells could compensate for the loss of embryonic nerve cells not only structurally but also functionally. Thus, chimeric mice were able to find hidden food in a more targeted manner using a sense of smell restored by rat cells. A mouse’s complex behavior was thus triggered by a rat’s sense of smell. The mouse smelled the food with a rat’s nose, so to speak.
Chimera research helps to generate knowledge in order to make cell and tissue replacement therapies available to patients more quickly. However, the chimera creation approach presented here is not an approach that can be directly transferred to humans as a therapy. The work presented here is very valuable for a better understanding of the embryonic development of brains, their evolutionary adaptations and how they function.
Accompanying bioethical research is necessary at the latest when human embryos are to be used as recipients in chimera research. Personally, I would strictly reject the use of human embryos in interspecies chimera research, even if this were permitted in Germany. But even if human stem cells are transplanted into animal embryos, which may make sense from a biomedical point of view, a biomedical-bioethical discourse should take place.
Modern chimera research is still in its infancy. Important fundamental findings can currently be obtained from practically every chimera study, which in their entirety strongly promote the concept of growing replacement organs from human cells for organ transplants, for example in pigs. In my opinion, the two studies that have now been published do not yet provide a translational approach for new therapeutic approaches. However, they contribute significantly to the scientific knowledge on which new therapies can be built in the long term.
Stefan Schlatt - quimeras EN
Stefan Schlatt
Director of the Centre of Reproductive Medicine and Andrology, Münster University Hospital (UKM), Germany
No cause for concern but cause for amazement. We have known for many years that cells injected into blastocysts can repair an organism. Now this has been confirmed by experimentally produced organ defects. It is hardly surprising that this also applies to the brain. However, it is quite astonishing that neuronal cells are extremely plastic and support organogenesis across species boundaries, including in the brain. Here we need to revise our understanding of genetics and species boundaries in favor of a greater significance of cellular plasticity in the formation of organs.
Chimeras allow us to study the interaction of different cells through markers. This is extremely helpful for describing developmental processes in organ formation. In these xenological studies on the brain, it becomes clear that neuronal cells can also correspond with each other across species boundaries and form functional networks. The fact that they adapt their repair program depending on the conditions (cells sick but present, cells eliminated) was previously unknown. In this respect, these studies will provide important findings for therapeutic approaches. Whether only the findings or even cells will actually be used for this purpose cannot be estimated at present.
In principle, such experiments are also possible between other species. However, we already know from other organ systems that regenerative processes are not arbitrarily interchangeable. For example, testicular stem cells from rats can generate sperm in the testicles of mice, but primate stem cells cannot. Although they settle, they do not differentiate and remain as stem cells in the mouse testis. It will be exciting to study the different organ systems and learn which processes are more conserved and allow interspecies communication and interaction.
For ethical reasons, caution seems to be called for here. While blastocyst complementation opens up a highly interesting field for basic research, the generation of organ replacements for clinical applications is not a realistic scenario. There still appears to be far too little knowledge about species- and organ-specific effects to be able to assess the risks. As with cloning, there should be an internationally recognized moratorium here.
Throesch et al.
- Research article
- Peer reviewed
- Animals
Huan et al.
- Research article
- Peer reviewed
- Animals