Global child immunisation levels stagnate in 2023, according to WHO and UNICEF data
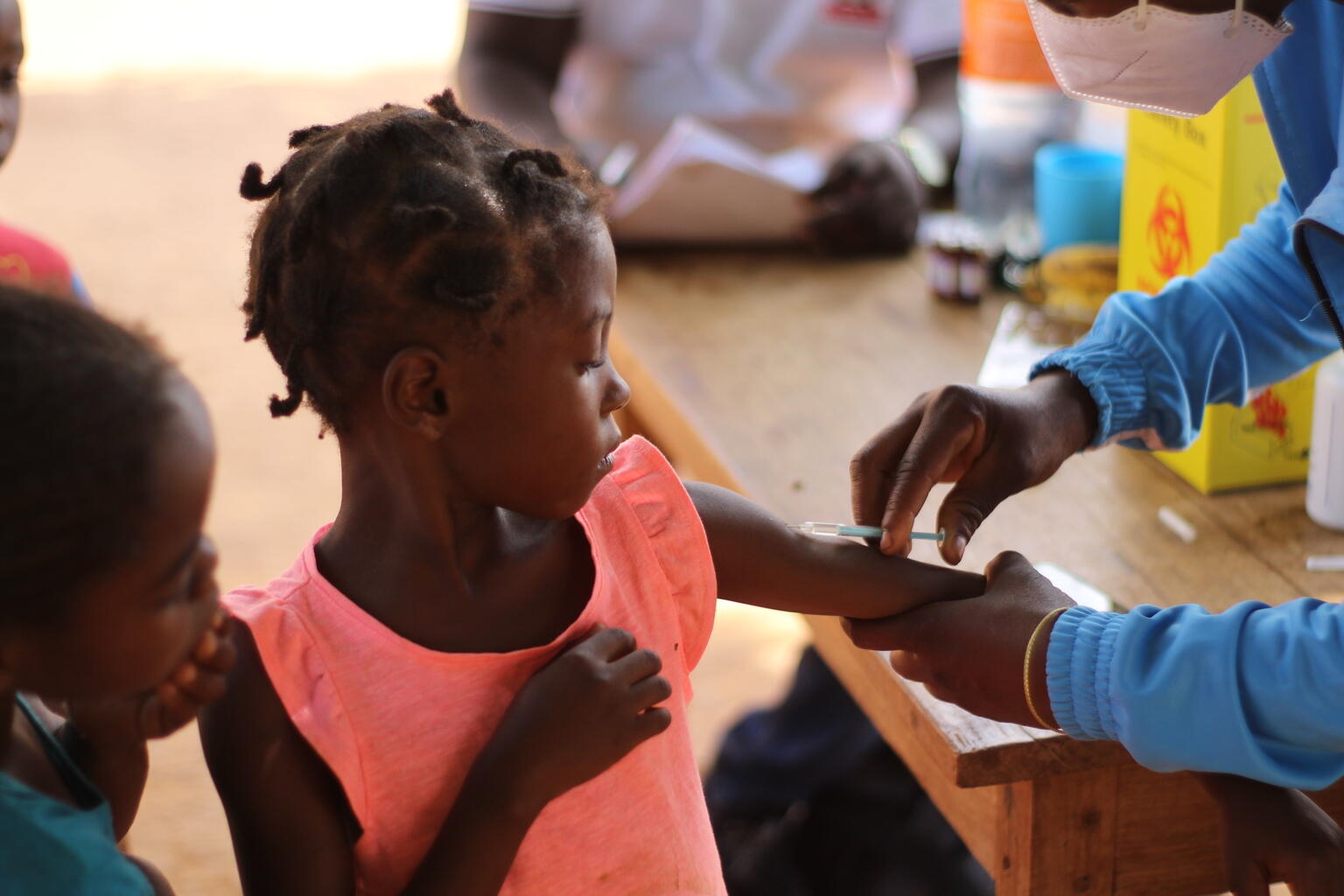
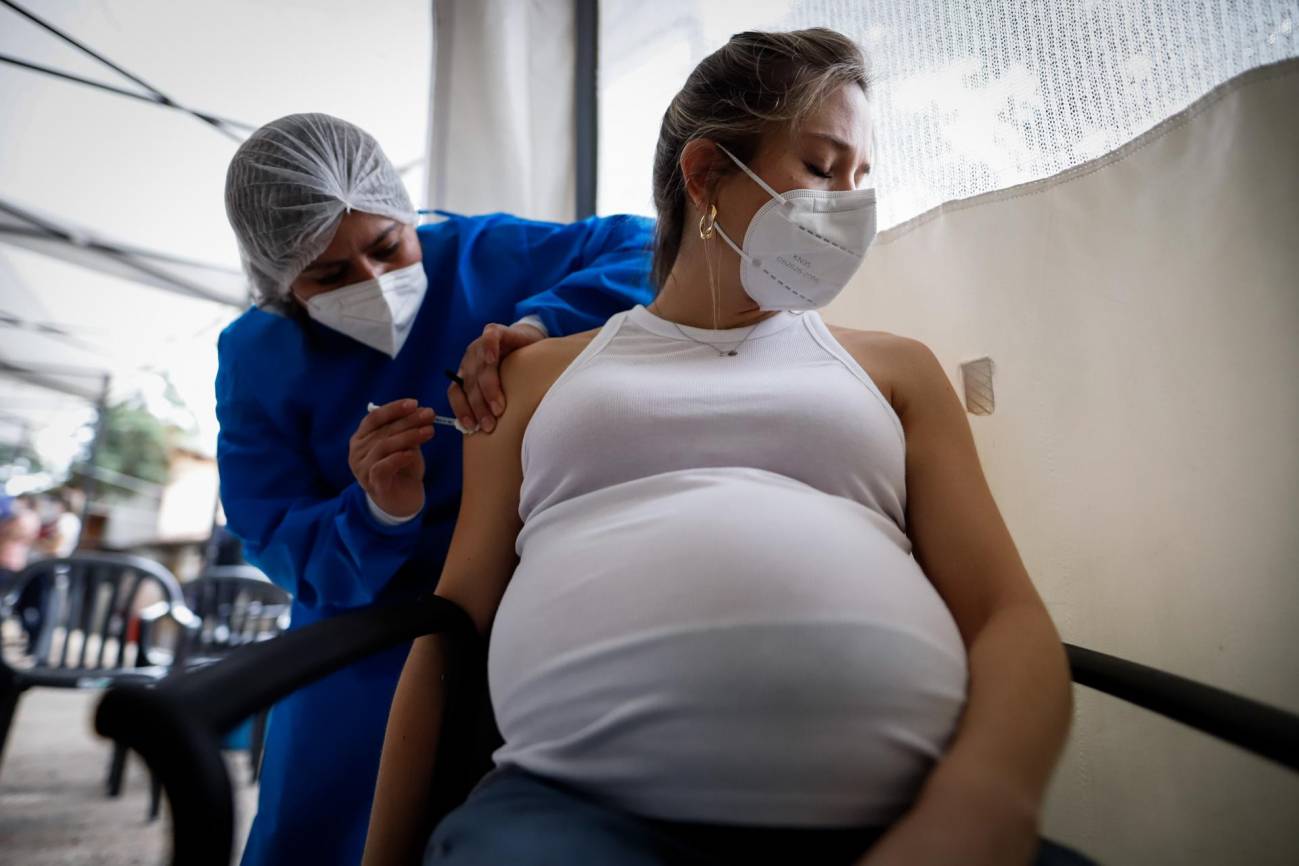
Global childhood immunisation coverage stagnated in 2023, with 2.7 million more children unvaccinated or under-vaccinated than at pre-pandemic levels in 2019. This is one of the data published by the World Health Organization (WHO) and UNICEF in the World Health Organization's Worldwide Estimates of National Immunisation Coverage (WUENIC), which captures global vaccination trends against 14 diseases. More than half of unvaccinated children live in 31 countries with fragile and conflict-affected environments.