Clinical trial demonstrates efficacy of RH5.1/Matrix-M malaria vaccine in babies in Burkina Faso
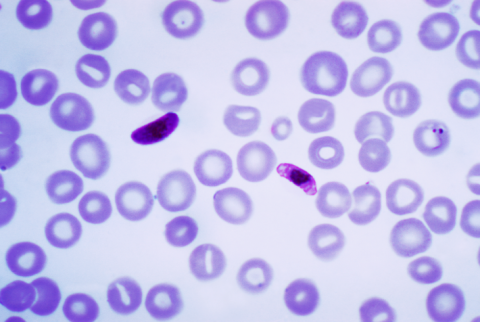
The RH5.1/Matrix-M vaccine is effective and safe against malaria, according to a phase 2b clinical trial in infants in Burkina Faso published in The Lancet Infectious Diseases. Two other vaccines are already approved against malaria, a disease caused by the Plasmodium falciparum parasite, but this one acts at a different stage of the disease: when the malaria parasite is present in the blood. The other two vaccines attack the parasite when reaches the liver.
241211 vacuna malaria - consuelo EN
Consuelo Giménez Pardo
Professor of Parasitology at the University of Alcalá (UAH) and director of the Master's Degree in Humanitarian Health Action (UAH-Doctors of the World)
Led by researcher Hamtandi Magloire Natama, interim results in children from a double-blind, randomised, controlled, double-blind phase IIb trial of RH5.1/Matrix M, a vaccine against blood-borne merozoites, are presented, which appears to provide a second line of paediatric defence against clinical malaria. Dr Magloire Natama - a researcher with expertise in the study of genetic and immunological factors contributing to inter-individual variation in susceptibility to malaria in early childhood - was also co-investigator and coordinator of the phase II trial of R21/Matrix-M, the second malaria vaccine after RTS, S/AS02, which is also pre-erythrocytic and capable of targeting another stage of the parasite's life cycle: sporozoites.
The proposed trial was conducted in a controlled study in children aged 5-17 months in the Nanoro region of Burkina Faso, administering the vaccine during the malaria transmission season. The paper, published in The Lancet Infectious Diseases, reports that RH5.1/Matrix M is safe and well tolerated, and in three doses provides anti-RH5.1 antibodies. The authors propose that, as a promising strategy in this second generation of paediatric malaria vaccines, it should be used synergistically in combination with existing pre-erythrocytic vaccines.
241211 vacuna malaria - carlota EN
Carlota Dobaño
Research professor and Head of the Malaria Immunology Group at the Barcelona Institute for Global Health (ISGlobal)
This is a randomised, controlled, double-blind clinical trial, which is the most robust methodology available in epidemiology, although the results are still preliminary from an interim analysis and a more precise estimate of the vaccine's efficacy must await the final analysis.
This is the first time that a malaria vaccine based on an antigen from the blood phase of the parasite (the one that causes the symptoms of the disease and pathology), specifically the Plasmodium falciparum Rh5 protein, together with the adjuvant already used in other vaccines (R21: MatrixM) and in covid-19, has shown significant efficacy in African children, without depending on the strain with which it was vaccinated. The vaccine is safe, generates high neutralising antibodies and is effective against malaria within six months at 55%, which is equivalent to what RTS,S showed at the same age in the phase 3 trial for registration. It also provides the option of varying (delaying) the time periods or vaccination intervals between doses to improve the potency of the vaccination.
The results need to be confirmed in other malaria endemic areas where transmission is not so markedly seasonal. In addition, the confidence intervals for vaccine efficacy are wide and therefore the estimate is not yet precise. Longer follow-up in children is still needed to assess the duration of protection since one of the main limitations of the current vaccines is their longevity over time, which needs to be improved to avoid the need for booster doses.
It is a promising vaccine to combine with the two existing malaria vaccines (RTS,S or R21) which are based on the pre-sanguineous or hepatic phases and do not fully prevent malaria infection. Therefore, the combination of both types of partially protective vaccines could lead to a more potent multivalent vaccine. It also indicates that delaying the third dose of vaccination could benefit the immune system response, resulting in better antibody response and greater efficacy.
For additional context, see our commentary on this group's previous work with this vaccine, which has not yet shown efficacy.
Hamtandi M Natama et al.
- Research article
- Peer reviewed
- Clinical trial
- People