Mice with fur reminiscent of extinct woolly mammoth created
Colossal Biosciences has announced the creation of genetically modified mice with characteristics reminiscent of the fur of extinct mammoths, such as fur colour, texture and thickness. The non-peer-reviewed results were shared today in the BioRXiv prepublication repository.

Lluís Montoliu - raton mamut
Lluís Montoliu
Research professor at the National Biotechnology Centre (CNB-CSIC) and at the CIBERER-ISCIII
While we wait to de-extinct a woolly mammoth, we already have a woolly mouse. Some researchers never miss a trick. They are capable of carrying out and completing the most fantastical and extravagant ideas we can imagine. Ideas that the rest of us dismiss as impossible or unfeasible. For these researchers, nothing is impossible. Their conviction that they have the answer to problems, their perseverance and stubbornness usually bring them successes that the scientific community applauds with a mixture of surprise and bewilderment. This is the case of George Church, Harvard geneticist, founder of numerous companies and visionary.
Church was the one who, in 2015, managed to eliminate each and every one of the 62 porcine retrovirus integrations in the pig genome that limited its use for xenotransplantation, using CRISPR gene editing tools. Church was also the one who, in 2017, encoded and stored five frames of the first film ever shot at the end of the 19th century (a galloping horse) in the genome of bacteria, also using CRISPR. And Church is also the one who, together with Ben Lamn, founded a company in 2014 with a name as overwhelming (Colossal) as the objective they set themselves: the de-extinction of the woolly mammoth, a pachyderm whose last individuals disappeared some 4,000 years ago from an island in northern Siberia. And with a justification no less surprising: to combat climate change.
And now he is announcing the creation of a woolly mouse with some of the mammoth's characteristics, detailed in a preprint not yet published in any peer-reviewed scientific journal. Church may surprise us again with this experiment. However, it has its logic, as I will try to explain below.
The closest living animal, evolutionarily speaking, to the woolly mammoth is the Asian elephant. Using DNA obtained from various mammoth carcasses that have remained frozen and relatively well preserved for thousands of years in the Siberian tundra, the company Colossal has been able to obtain a high-quality mammoth genome to compare with that of the Asian elephant. There are around 500,000 changes between the two genomes that Church and his colleagues want to incorporate, one by one, using CRISPR tools, using cultured Asian elephant cells as starting material. This will take some time, and it is only the first of the technical challenges they will have to solve. Then they will have to reconstruct mammoth embryos using Asian elephant eggs and nuclei from the edited cells by means of nuclear transfer (cloning) and gestate them, probably in some extra-uterine system that has yet to be invented, improving on the existing systems that allow gestation and growth to be maintained outside the maternal uterus in lambs and premature babies. All this is going to take a long time, impossible to predict. And that is why they need intermediate successes that justify their project and allow them to move forward, demonstrating, with much simpler experiments, that it is possible to edit the genome of an animal to incorporate selected characteristics.
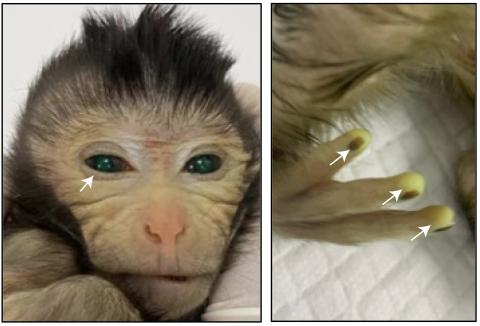
Church, together with the Colossal researchers, has applied different versions of CRISPR gene editing technology (both the first-generation tools, initially described by Emmanuelle Charpentier and Jennifer Doudna, as well as second-generation tools, the base editors, described by David Liu) on various mouse genes (up to 10), modifying or inactivating them, reproducing some of the genetic variants found in the mammoth that allowed it to withstand the freezing temperatures of its time. They have also resorted to a classic strategy of gene inactivation in embryonic pluripotent stem cells. All these approaches, in parallel, have allowed them to generate numerous mice, with different combinations of edited genes (the mouse that accumulates the most mutations has seven edited genes), which show an appearance and characteristics reminiscent of the woolly mammoth.
Thus, the inactivation of the Mc1r gene changes the dark color of the hair and turns it yellowish, reddish, like red-haired people and animals, like the mammoth had. Inactivation of the Fgf5 gene causes hair to grow up to three times longer than normal. Inactivation of the Fam83g, Fzd6, Tgm3, Astn2, Krt25, Tgfa and Krt27 genes alters the pattern of hair growth, which begins to curl and become thicker, as it did in the mammoth. The final appearance of the mouse thus edited is that of a woolly mouse, with thick, long, curly, red hair, similar to that of the mammoth, and surely much better prepared to withstand low temperatures than unmodified wild mice.
They have also edited the Fabp2 gene, which is involved in lipid metabolism and is thought to contribute to the storage of enough body fat to insulate and nourish the mice during the long winters. So far, the furry mice with this latest gene do not yet accumulate more weight than their unmodified siblings, which suggests that other gene modifications will be necessary for them to acquire this characteristic.
Naturally, each editing experiment carries a certain risk of uncontrolled genetic modifications in other similar places in the genome, a problem that these researchers have tried to limit as much as possible by selecting very specific RNA guides that only direct CRISPR editing in the intended genes. However, when editing many genes at once, these risks are also multiplied. Something they will have to take into account when they have to tackle not a dozen but thousands of genetic edits, to convert the genome of an Asian elephant into that of a mammoth.
In short, this experiment reported by Church and the researchers from the company Colossal demonstrates their ability to introduce precise genetic changes in a mouse, derived from the genetic differences found in the mammoth genome. No, they haven't yet de-extinct any mammoths, but they have shown the effect of some genetic variants found in the mammoth genome using mice as an experimental system to validate the effects produced by these mutations. A new indisputable success for Church that once again demonstrates his technical excellence, leaving both his followers and his critics speechless.
Alena - ratones lanudos EN
Alena Pance
Senior Lecturer in Genetics, University of Hertfordshire
Genetic engineering in mice has been performed for a very long time using, developing, and testing a variety of technologies. These modifications include introducing traits from other species, notoriously ‘humanised mice’ that have been used for research related to human traits and disease.
The decoding of an extinct species genome to identify specific genes associated with particular traits has also been done before, where the physical characteristics of ancient humans have been deduced from their genomic data.
Perhaps the novelty here is using mice to confirm the assumptions about correlations between genes and specific traits. The press release gives the impression that mammoth genes were introduced to mice but from the preprint, it transpires that the genomic editing in these mice consists of inducing loss of function of several genes simultaneously. The choice of these genes comes from observed spontaneous mutations in mice that impact traits such as coat and comparative analyses of elephant and mammoth genomes that reveal similar loss of function in some of these genes.
The ability to use mice in order to examine and test gene-trait relationships and hypotheses about physical characteristics specifically using genomes from extinct organisms might prove useful, but overall not particularly novel.
Denis - ratones lanudos EN
Denis Headon
Group Leader and Senior Research Fellow, The Roslin Institute, University of Edinburgh
With a long-term goal of advancing the de-extinction of the mammoth, the team have managed to alter several mouse genes in one step. They chose these gene alterations based largely on things that we know about mice, rather than what we know about mammoths. This approach produced very shaggy mice with a coat that resembles that of the woolly mammoth remains we find today. While the mice have a striking golden coat, they are otherwise healthy, indicating that the method used is not harmful. Certainly this is an advance in speeding up the rate of genetic modification towards the many changes that distinguish one species from another, though it’s not clear that these changes alone would alter a relatively hairless elephant into a woolly animal. Further work on either synthesising or understanding the mammoth genome would also be required to go beyond these superficial characteristics to generate an animal that would, for example, have the right behaviour to live in Arctic conditions. This paper reports an important advance not only for de-extinction but for animal breeding in general.
Louise - ratones lanudos EN
Louise Johnson
Evolutionary Biologist, University of Reading
Seeing these mice is a bit like looking back at the past, but with a highly selective telescope. This technology offers an exciting opportunity to test some of our ideas about extinct organisms.
It is an interesting piece of work, but the idea that we could bring something back from extinction is false hope.
What has been done here is not trivial, but of the ten different mutations engineered into the mice, only a few actually make the mouse gene closer to a known mammoth gene. The result does show that it is possible to genetically engineer many genes at once and still produce some live mice at the end of the process, though. The researchers have succeeded in nudging the mouse genome in the direction of a mammoth genome, which is a first.
If we have an idea of what a gene might do in an extinct mammal, this technology can produce powerful results by introducing a very similar sequence into a mouse. But in this particular case, most of the mutations are chosen just because they are already known to make mice have longer, coarser, wavier hair. You could, in theory, produce mice like this by just breeding mice with weird hair together.
In theory, you could introduce a gene for hairiness into an elephant and it would look quite mammoth-like, but it wouldn’t be a mammoth in any meaningful way. Elephants would be a terrible species to do this research with – they are huge, have long gestation periods, and require highly specialist housing and care. The mouse is a brilliant lab animal, and we know a lot about the mouse genome and how to alter it effectively.
You do have to know a bit about how the extinct genes might work. For example, it was already known that the genes for coat colour and texture were similar in the mammoth and the mouse. Being able to create and introduce a mouse gene that is somewhat the same as the mammoth opens up a new way to look at evolutionary genetics.
Tori Herridge - ratones lanudos EN
Tori Herridge
Senior Lecturer, School of Biosciences, University of Sheffield
Woolly Mouse in Context
“Colossal have announced that they have successfully bred ‘woolly mice’, and this is a “water shed moment” in their mission to genetically engineer an arctic adapted elephant, aka “bringing back the mammoth.”
“Colossal’s team made a number of genetic changes known as “knock outs” in lab mice that are already known to produce longer, thicker, wavier -- or woollier -- coats in mice. They also made a change known to cause blonde hair colouring in mice.
“The result, therefore, of various “woolly mice” from these genetic changes is unsurprising: woolly mice have been produced in labs and by mice breeders many times before.
Mammoth-like genes?
“Three of the genetic changes made in some of the mice were inspired by woolly mammoth DNA, but they still only show effects in mice. The mice were not edited to have a precise copy of the mammoth genes, but it is possible that these edits may have had a similar effect in both mice and mammoths (either by stopping the gene from working, or by changing the way the gene worked), but we cannot be sure about this.
“It is also not possible to tell what impact these ‘mammoth-inspired’ changes had, if any, in the Colossal woolly mouse owing to other gene edits made at the same time.
Are we a step closer to ‘bringing back the mammoth’?
“A mammoth is much more than just an elephant in a fur coat. While we know a lot about mouse genetics, we know much less about mammoths and elephants. It isn’t yet known which sections of the genome are vital for achieving the characters need to make an elephant fit for life in the Arctic circle. Genes that are linked to fur and fat in well-studied animals like mice are obvious targets, but the devil is in the detail. And what about other characters that are equally important? Which bits of the genome underpin the teeth and jaw changes that might be needed to accommodate an Arctic diet, for example (mammoth teeth were clearly under strong evolutionary pressure to adapt to their diet)? What about things we haven’t even discovered yet, things we don’t know we don’t know?
“Unless you decide to make EVERY edit necessary to in the genome, you are only ever going to create a crude approximation of any extinct creature, based on an incomplete idea of what it should look like. You are never going to ‘bring back’ a mammoth.
“Colossal’s Woolly Mouse experiments also show that de-extinction attempts are fraught with failure: most gene-edited embryos failed to result in live pups (less than 10%), and very few of those born were successfully edited for all target genes. This is for experiments that made a small number of relatively simple (loss of function) changes in well understood genes, using a ‘model’ lab animal as a surrogate.
“Engineering a mammoth-like elephant presents a far greater challenge: the actual number of genes likely to be involved is far higher, the genes are less well understood (and still need to be identified), and the surrogate will be an animal that is not normally experimented upon. Even if success rates are similar to those observed in the woolly mice (and they may well be lower given the greater number of edits and unknowns), there will likely need to be multiple pregnancies before a “successful” calf is born. This equates to either a very large number of surrogate dams, or – given elephant pregnancies last approximately 2-years – a very long time.
“Mammoth de-extinction doesn’t seem to be on the horizon anytime soon.
Ethical Considerations
“Colossal’s Woolly Mouse experiments show that the physical effect of genome-editing cannot be observed until the animal experimentation stage. This will also be true in elephants.
“Although it is branded as “woolly mammoth de-extinction”, what is being proposed is an experiment to test the effect of certain gene edits on the appearance of elephants.
“For the mice in these experiments the risk was small: the effect of these gene edits already known, and were not likely to cause risk or suffering to surrogate or pup.
“We do not know the risk involved for elephants, but it could be very high.
“We do know that surrogacy is a burden on the dam, and that captive elephant pregnancies carry risks even under normal circumstances.
“Placing such a burden of risk on an elephant surrogate in pursuit of an experiment that – at best – will produce a simulacrum of a woolly mammoth, is unjustifiable.
Chen et al.
- Research article
- Non-peer-reviewed
- Preprint
- Animals